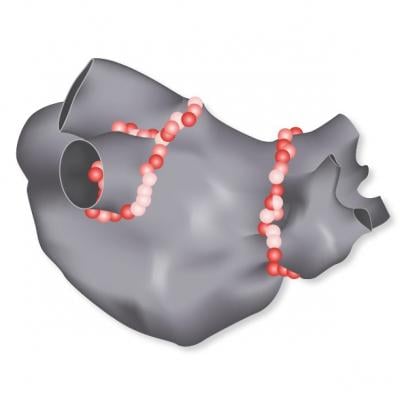
October 9, 2018 — Biosense Webster Inc. recently received approval from the U.S. Food and Drug Administration (FDA) for its Visitag Surpoint External Processing Unit, and enrollment has begun in its post-market approval study.
The Visitag Surpoint Module calculates a Tag Index, a single value combining parameters of power, contact force and duration during a catheter ablation procedure. The index was developed to simplify and standardize the workflow for ablating patients with paroxysmal atrial fibrillation (PAF) and to support electrophysiologists using the Carto Smarttouch Technology, to achieve pulmonary vein isolation (PVI).
Jose Osorio, M.D., at Grandview Medical Center in Birmingham, Ala., recently enrolled and treated the first patients in a post-market approval study that is assessing Tag Index-guided ablation using the Visitag Surpoint Module.
“We’re always looking for ways to improve procedure efficiency and are proud to be early adopters of this innovative technology,” said Osorio, board certified cardiac electrophysiology and cardiovascular disease, Grandview Medical Center. “I look forward to integrating the prescriptive Tag-Index guided ablation into my workflow for PVI.”
Over 30,000 patients have been treated with the technology outside the U.S, where it is commercialized as CARTO 3 System CARTO VISITAG Module with Ablation Index.
An estimated 33 million people worldwide have been diagnosed with atrial fibrillation and its prevalence is projected to increase significantly as the population ages.[1] Approximately 70 percent of patients with atrial fibrillation are between the ages of 65 and 85.[2]
For more information: www.biosensewebster.com
References


 February 06, 2026
February 06, 2026 









