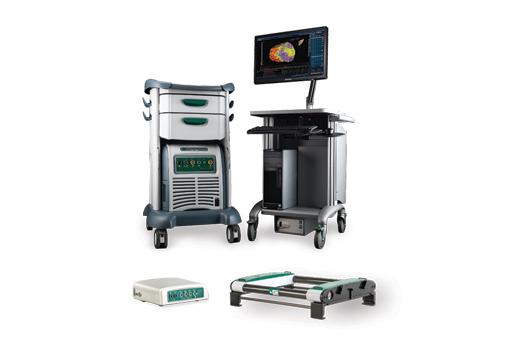
April 19, 2016 — St. Jude Medical Inc. announced expansion of its EnSite Precision cardiac mapping system limited market release in Europe. The company also noted use of the new platform in more than 600 cases in nine countries since receiving CE Mark in January 2016.
The mapping system is a next-generation platform designed to provide automation, flexibility and precision for tailored treatment in patients with cardiac arrhythmias. The launch constitutes introduction of an entirely new suite of technologies including innovations in hardware, catheters, surface electrodes (patches) and software features designed for improved ease of use.
The system is currently available and expanding in select European countries as part of its initial launch, and the company is currently pursuing U.S. Food and Drug Administration (FDA) clearance of the EnSite Precision cardiac mapping system.
The system is used in ablation procedures to visualize and navigate catheters in the heart. It provides highly detailed anatomical models and maps to enable diagnosis of a wide range of arrhythmias, guide therapy and provide expanded procedural options to tailor care for patients. During this initial launch, the EnSite Precision cardiac mapping system was used in a variety of procedures, from simple to complex, including atypical flutter, paroxysmal and persistent atrial fibrillation (AF) and ventricular tachycardia (VT), with excellent system performance through challenging procedure dynamics.
"The EnSite Precision system is precise, reliable, accurate and it's fast. This is very important when it comes to the demands of today's electrophysiologists, especially as we see more patients with complex substrate-based arrhythmias,” said Gerhard Hindricks, M.D., director, Department of Electrophysiology, University of Leipzig Heart Center in Germany. “This is really what we need to push clinical electrophysiology forward to a better level of successful case execution.”
The EnSite Precision cardiac mapping system uses intelligent automation tools to enable faster, more accurate high-density maps with greater consistency across cases. The system is flexible and enhances efficiency by allowing physicians to map heart chambers with any electrophysiology catheter and customize procedures to address the circumstances of each case. The system allows catheter navigation to occur with less fluoroscopy, thus reducing potential for risks associated with excessive radiation exposure.
For more information: www.sjm.com


 January 29, 2026
January 29, 2026 









