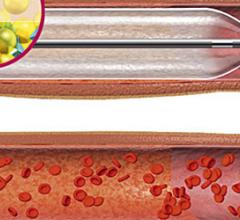
February 11, 2013 — Vascular Nanotransfer Technologies (VNT) says it has developed the industry’s most versatile drug ...
Balloon Catheter
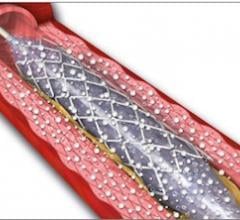
This channel includes news and new technology innovations for angioplasty balloon catheters (PTA). These are used in arteries with atherosclerotic lesions to compress the plaque expand the artery lumen to reopen occluded or heavily stenosed atherosclerotic lesions. Balloons are often used in combination with a stent to prop the treated vessel segment open. In addition to plain old balloon angioplasty (POBA), this section includes news about drug-coated balloon (DCB), valvuloplasty balloons and specialty cutting balloon.
To help identify trends and find out what DAIC readers are interested in, the magazine takes note of what they click on ...
January 28, 2013 — AngioScore Inc. announced the launch of its new 100 mm length AngioSculpt PTA Scoring Balloon ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...

January 22, 2013 — Using tiny balloons to temporarily block blood flow to the uterus during a high-risk Caesarean ...
December 26, 2012 — Covidien announced a definitive agreement to acquire CV Ingenuity. The companies expect to complete ...
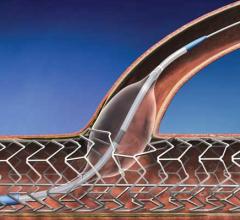
November 1, 2012 — It looks as though drug-eluting balloons (DEBs), already widely used in Europe, are set to burst into ...
October 17, 2012 — AngioDynamics entered into a definitive agreement to acquire all the outstanding capital stock of ...
September 28, 2012 — Boston Scientific Corp. announced it has received U.S. Food and Drug Administration (FDA) clearance ...
September 21, 2012 — Following the success of Biotronik’s Pulsar-18 0.018-inch, 4 French platform self-expanding stent ...
September 21, 2012 — Cardionovum GmbH announced that the results of a preclinical study and a first-in-man clinical ...
Austin Medical Design's Cath Cube is a single-use product for management of guidewires and catheters (balloons and ...
July 27, 2012 — C. R. Bard Inc. announced that its Lutonix technology center has completed patient enrollment into its ...
July 16, 2012 — TriReme Medical Inc. (TMI) announced that it has received U.S. Food and Drug Administration (FDA) ...
July 9, 2012 — r4 Vascular Inc. announced clearance from the U.S. Food and Drug Administration (FDA) to market the ...
July 9, 2012 — Miracor Medical Systems GmbH announced that the first three ST segment elevation myocardial infarction ...

 February 11, 2013
February 11, 2013