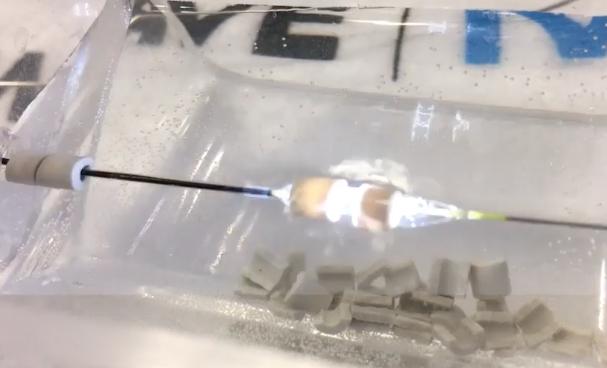
The Shockwave intravascular lithotripsy system produces a flash of light as it releases a sonic shockwave, as seen here as it is about to shatter a gypsum bead during a TCT 2019 demonstration at the vendor's booth. You can see the cracks forming as the bead breaks apart in the right bead. The technology combines a miniaturized lithotripsy system with a low-pressure balloon catheter to expand peripheral or coronary arteries without causing vessel trauma. Photo by Dave Fornell
After covering cardiovascular technologies for 14 years, it is rare that I find a new technology that might actually be a paradigm shift in cardiology. Most new devices I see are just refined iterations of existing devices, or the vendor added a new button or set of measurement tools. However, I do occasionally find a true gem while sitting in on hours of new technology sessions at conferences, or while wandering the expo floors.
One such gem that may soon become a paradigm shift just gained U.S. Food and Drug Administration (FDA) clearance to treat calcified coronary arteries. The Shockwave Medical Intravascular Lithotripsy uses sonic wave pulses and a low pressure 4 atm compliant balloon to break up heavy calcium without vessel trauma. Vessel trauma is currently the only way to break up these lesions to prep them for stenting using either high pressure balloons, cutting balloons or atherectomy. I first saw this catheter technology break egg shells set inside gelatin as a bench test video during a new futuristic innovations session at the Transcatheter Cardiovascular Therapeutics (TCT) 2013 meeting. I closely followed this technology ever since because of its possibility to be disruptive in interventional cardiology. At later TCT meetings, I saw preclinical and later clinical fluoro imaging of heavily calcified peripheral leg lesions magically reopen in seconds using this technology.
Intravascular lithotripsy gained FDA clearance for peripheral artery disease (PAD) in 2016, and the company began working toward an additional indication for the coronaries. It was granted FDA clearance in February 2021. The FDA also recognized this technology as a breakthrough technology because it offers a solution to a long-standing clinical problem.
The FDA pivotal trial for the coronary indication was presented as a late-breaker at TCT 2020 by Dean Kereiakes, M.D., FACC, FSCAI, president of The Christ Hospital Heart and Vascular Institute. He said this technology is a paradigm shift. Hear more on what he had to say about the data and the technology in the VIDEO: Intravascular Lithotripsy to Treat Severely Calcified Coronary Artery Lesions.
Lastly, when looking at new technologies, I always consider how practical it is to use. I have seen a lot of really interesting technologies over the years, but some were just too cumbersome or too difficult to use or interpret, or did not offer enough of an advantage over the current standard of care. In evaluations for practicality, I use the gauge that if it is something I would feel comfortable using after a 15 minute tutorial (and keep in mind I am not a cardiologist) it will probably see wide-spread use in cath labs. Shockwave’s intravascular lithoplasty is one of these technologies that is very easy to use and it solves an age-old problem of how to build a better mouse trap in dealing with coronary calcium.
Here is a a very quick demo of the technology I shot on my iPhone on the expo floor of TCT 2019 where it quickly shatters gypsum beads — VIDEO: Demonstration of Intravascular Lithotripsy Breaking Up Calcium.
Here are other articles and video interviews on this technology from the past few years DAIC followed it through pre-clinical, clinical and regulatory reviews.
FDA Clears Coronary Intravascular Lithotripsy to Breakup Calcified Lesions With Sound Waves
VIDEO: Clinical Example of Coronary Lithotripsy to Break up Calcified Lesion
Intravascular Lithotripsy: Will This New Investigational Technology Crack Calcium’s Code in the U.S.? — Article by DISRUPT CAD III investigators Dean Kereiakes, M.D., and Jonathan Hill, M.D.
FDA Grants Shockwave Medical Breakthrough Status for Coronary Intravascular Lithotripsy
VIDEO: Breaking Up Calcified Lesions Without Vessel Trauma — Interview with Todd Brinton, M.D.
FDA Clears Lithoplasty Balloon That Shatters Calcified PAD Lesions With Ultrasound
Lithotripsy Safe and Effective in Calcified Stenotic Peripheral Arteries
Intravascular Lithotripsy May Offer Solution for Calcified Coronary Lesions — Article By Azeem Latib, M.D.
VIDEO: How a Lithoplasty Balloon Shatters Calcified Plaque in Arteries With Ultrasound



 November 14, 2025
November 14, 2025 









