December 4, 2018 — New results from the DISRUPT PAD II study showed no perforations, embolization, reflow or abrupt closures with the Shockwave Peripheral Intravascular Lithotripsy (IVL) System in the treatment of calcified, stenotic, peripheral arteries. Results of the non-randomized, multi-center study were published in Catheterization & Cardiovascular Interventions (CCI).1
The results were the first safety and performance report of the stand-alone IVL therapy in calcified femoropopliteal lesions with 12-month follow-up. The trial is also noteworthy in that it included a highly complex patient population with a severely calcified superficial femoral artery (SFA) and popliteal lesions that are typically excluded from contemporary peripheral studies.
Other notable findings of the study included:
- 1.7 percent grade D or higher dissection (guidewire-induced);
- 24 percent residual stenosis with IVL alone;
- 100 percent procedural success; and
- Less than 9 percent target lesion revascularization (TLR) at one year when optimal technique was employed (balloon oversizing and segment overlap).
Watch the VIDEO "Breaking Up Calcified Lesions Without Vessel Trauma."
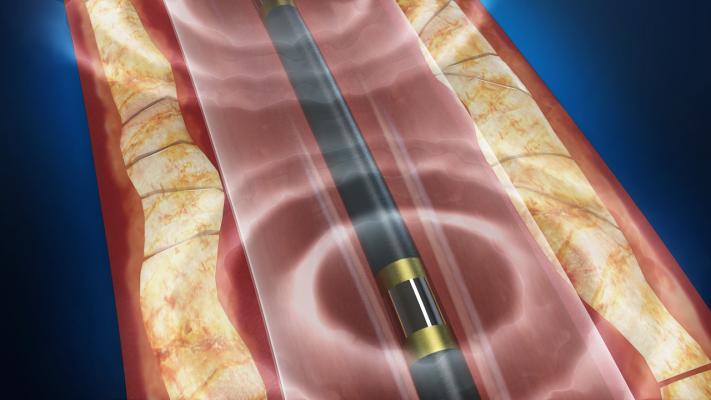
IVL is a novel therapy designed to treat calcified artery blockages with sonic pressure waves historically used to treat patients with kidney stones. The technology minimizes trauma within the artery by delivering pulsatile sonic pressure waves locally to effectively fracture both intimal and medial calcium in the artery wall but pass through surrounding soft vascular tissue in a safe manner. Additionally, IVL requires no specialized training, and it allows physicians to use their own guidewire of choice to seamlessly integrate into the existing workflow.
Watch the VIDEO How a Lithoplasty Balloon Shatters Calcified Plaque in Arteries With Ultrasound
The Shockwave IVL System has achieved other notable milestones in the last 18 months, including the first commercial treatment case in the United States and initiation of the DISRUPT PAD III study. DISRUPT PAD III will assess the optimal therapy to dilate heavily calcified lesions by comparing the Shockwave Medical Lithoplasty System versus traditional angioplasty, with a primary goal of achieving less than 30 percent residual stenosis without the need for stenting. In addition, all patients who do not receive a stent will be treated with a drug-coated balloon. The trial, which was initiated in June 2017, will enroll 334 patients in up to 45 global sites.
Read the article First U.S. Patient Treated in DISRUPT PAD III Study of Lithoplasty Technology
For more information: www.shockwavemedical.com
Reference
1. Brodmann M., Werner M., Holden A., et al. Primary outcomes and mechanism of action of intravascular lithotripsy in calcified, femoropopliteal lesions: Results of Disrupt PAD II. Catheterization & Cardiovascular Interventions, Nov. 25, 2018. https://doi.org/10.1002/ccd.27943


 January 05, 2026
January 05, 2026 









