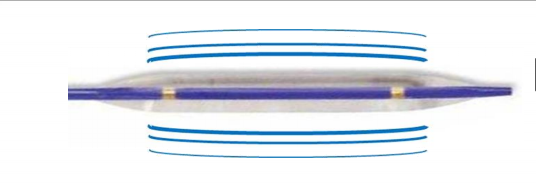
September 3, 2019 — Shockwave Medical Inc. has received Breakthrough Device Designation from the U.S. Food and Drug Administration (FDA) for its Shockwave intravascular lithotripsy (IVL) system with the Shockwave C2 Coronary IVL Catheter. The device is currently the subject of an Investigational Device Exemption (IDE) study called DISRUPT CAD III. The Shockwave C2 IVL Catheter is a proprietary tool designed to fracture problematic calcium using sonic pressure waves in order to facilitate stent delivery, deployment and optimal expansion, thereby improving blood flow to the heart muscle.
The FDA Breakthrough Device Program is intended to help patients and healthcare providers receive more timely access to medical devices that have the potential to provide more effective treatment or diagnosis for life-threatening or irreversibly debilitating diseases or conditions. Under the program, the FDA will provide Shockwave Medical with priority review and interactive communication during the Shockwave C2 IVL Catheter premarket review phase.
Coronary artery calcium physically impairs blood flow and restricts artery dilation, which inhibits stent expansion1 and is perhaps the single most important predictor of restenosis and early stent thrombosis,2 or coronary artery re-narrowing and blood clots, within the stent after-stent procedures.
DISRUPT CAD III is a prospective, non-randomized, multicenter global IDE study to demonstrate the safety and effectiveness of the Shockwave IVL System with the Shockwave C2 Coronary IVL Catheter in de novo, calcified, stenotic, coronary arteries prior to stenting. The study is approved to enroll 442 patients at 50 centers in the United States and Europe, and is led by co-principal investigators Dean Kereiakes, M.D., and Jonathan Hill, M.D. As reported in the company’s second quarter earnings call, the study had enrolled 108 patients as of June 30, 2019.
Shockwave C2 Coronary IVL catheters are commercially available for the treatment of de novo coronary artery disease in Europe and select other geographies; they are limited to investigational use in the United States.
For more information: www.shockwavemedical.com
Related Content
VIDEO: Breaking Up Calcified Lesions Without Vessel Trauma
FDA Clears Lithoplasty Balloon That Shatters Calcified Lesions With Ultrasound
References
1. Chambers J.W., Feldman R.L., Himmelstein S.I., et al.Pivotal trial to evaluate the safety and efficacy of the orbital atherectomy system in treating de novo, severely calcified coronary lesions (ORBIT II). Journal of the American College of Cardiology: Cardiovascular Interventions, May 2014. doi: 10.1016/j.jcin.2014.01.158.
2. Généreux P., Madhavan M.V., Mintz G.S., et al. Ischemic outcomes after coronary intervention of calcified vessels in acute coronary syndromes. Pooled analysis from the HORIZONS-AMI (Harmonizing Outcomes With Revascularization and Stents in Acute Myocardial Infarction) and ACUITY (Acute Catheterization and Urgent Intervention Triage Strategy) TRIALS. Journal of the American College of Cardiology, May 2014. doi: 10.1016/j.jacc.2014.01.034. Epub 2014 Feb 19.


 November 14, 2025
November 14, 2025 









