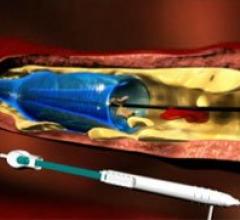
November 15, 2011 – Results from the PROFI study indicate that the use of a proximal balloon occlusion in carotid artery ...
Balloon Catheter
This channel includes news and new technology innovations for angioplasty balloon catheters (PTA). These are used in arteries with atherosclerotic lesions to compress the plaque expand the artery lumen to reopen occluded or heavily stenosed atherosclerotic lesions. Balloons are often used in combination with a stent to prop the treated vessel segment open. In addition to plain old balloon angioplasty (POBA), this section includes news about drug-coated balloon (DCB), valvuloplasty balloons and specialty cutting balloon.
November 14, 2011 — A clinical trial of patients with restenosis in drug-eluting stents (DES) in native coronary ...
November 14, 2011 – Boston Scientific welcomed positive outcomes from the COBRA clinical trial, which evaluated post ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
November 14, 2011 – Medrad Inc. announced that five-year data from the THUNDER trial [1] demonstrated a 59 percent ...
November 4, 2011—Miracor Medical Systems GmbH announced that data will be reported during next week’s Transcatheter ...
October 7, 2011 — Among individuals 65 years and older, as many as 30 percent have aortic valve sclerosis or stenosis ...
September 20, 2011 — Boston Scientific Corp. has launched its Coyote Balloon Catheter, a highly deliverable and ultra ...
September 15, 2011 – The Cardiovascular Research Foundation (CRF) has announced the late breaking trials and first ...
September 15, 2011 — Very positive results were reported in the first account of a clinical study evaluating the ...
September 9, 2011 — Angioslide Ltd. announced the first procedures with its new 5 x 300 mm Proteus device for treating ...
August 5, 2011 — Vessix Vascular Inc. (formerly known as Minnow Medical Inc.), developer of novel percutaneous ...
July 26, 2011 – The first patient has been enrolled in the LEVANT 2 clinical trial, a global, multi-center, randomized ...
June 20, 2011 — Boston Scientific Corporation today announced the global launch of its Mustang PTA balloon catheter, a ...
May 24, 2011 - Cordis announced it has obtained CE mark for its Empira and Empira NC RX percutaneous transluminal ...
May 17, 2011 - Medrad Inc., a business of Bayer HealthCare, today announced it has received CE Mark for its Cotavance ...

 November 15, 2011
November 15, 2011