Take a video tour of some of the medical devices designed to improve patient care, improve patient engagement and ...
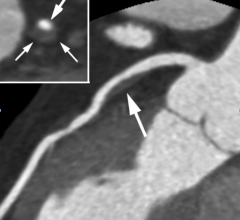
January 26, 2017 — Results from a core lab analysis from the PROMISE Trial on cardiac computed tomography (CT) were ...
January 26, 2018 — LivaNova PLC and MicroPort Scientific Corp. announced the companies have entered into a binding ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
Medtronic plc announced the initiation of its investigational device exemption (IDE) study for the Abre venous self-expanding stent system. The ABRE IDE Study will evaluate the safety and effectiveness of the Abre stent in subjects with iliofemoral venous outflow obstruction. The first procedure was performed in December of 2017, by Erin Murphy, M.D., director of the venous and lymphatic program at Carolinas HealthCare System’s Sanger Heart & Vascular Institute in Charlotte, N.C., and national principal investigator for the ABRE IDE Study in the United States.
January 25, 2018 – Modalixx G202MDL is a new grayscale High Bright medical LCD solution for modality CRT monitor ...
January 25, 2018 – Canon Medical Systems, in partnership with Quality Electrodynamics (QED), installed a Vantage Galan ...
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
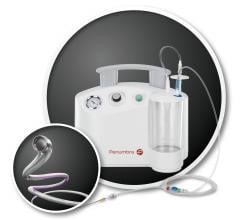
January 25, 2018 – Penumbra Inc. today announced results of the company-sponsored PROMISE Study, demonstrating real ...
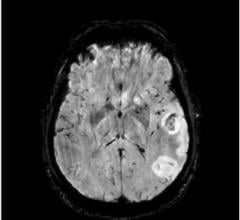
January 25, 2018 – Advances in brain imaging can identify a greater number of stroke patients who can receive therapy ...
January 25, 2018 – According to the latest market study released by Technavio, the global circulatory support devices ...
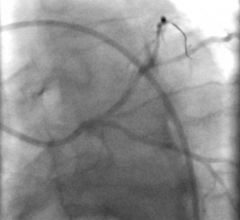
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
January 25, 2018 – The Intersocietal Accreditation Commission (IAC) announced the release of its Cardiovascular ...
January 25, 2018 – Data presented at the Biotronik-sponsored symposium on the Orsiro drug-eluting stent (DES) ...
January 25, 2018 – Elixir Medical Corporation, a leader in the development of breakthrough adaptive remodeling ...
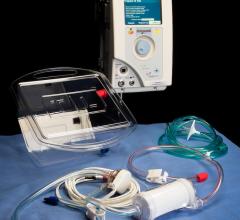
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...
January 25, 2018 – In a large population study that was the first of its kind, researchers found that a simple tool not ...
Cardiovascular Systems Inc. recently announced two new partnerships broadening the company’s product portfolio. CSI is now the exclusive U.S. distributor of OrbusNeich balloon products. Additionally, the company has signed an original equipment manufacturer (OEM) agreement with Integer Holdings Corp. for CSI-branded Zilient guidewires.
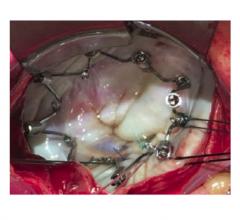
Boston Scientific Corp. announced it has closed an investment and entered into an acquisition option agreement with Millipede Inc., a privately-held company that has developed the IRIS Transcatheter Annuloplasty Ring System for the treatment of severe mitral regurgitation (MR). Under the terms of the agreements, Boston Scientific has purchased a portion of the outstanding shares of Millipede along with newly issued shares of the company for a total consideration of $90M. Boston Scientific has the option to acquire the remaining shares of the company at any time prior to the completion of a first in human clinical study that meets certain parameters. Upon the completion of the clinical study, Millipede has the option to compel Boston Scientific to acquire the remaining shares of the company. Each company's option period expires by the end of 2019. Completion of this acquisition would result in an additional $325M payment by Boston Scientific at closing with a further $125M becoming payable upon achievement of a commercial milestone.

 January 29, 2018
January 29, 2018