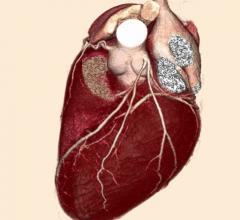
January 25, 2018 – The Intersocietal Accreditation Commission (IAC) announced the release of its Cardiovascular ...
January 25, 2018 – Data presented at the Biotronik-sponsored symposium on the Orsiro drug-eluting stent (DES) ...
January 25, 2018 – Elixir Medical Corporation, a leader in the development of breakthrough adaptive remodeling ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
January 25, 2018 – In a large population study that was the first of its kind, researchers found that a simple tool not ...
Cardiovascular Systems Inc. recently announced two new partnerships broadening the company’s product portfolio. CSI is now the exclusive U.S. distributor of OrbusNeich balloon products. Additionally, the company has signed an original equipment manufacturer (OEM) agreement with Integer Holdings Corp. for CSI-branded Zilient guidewires.
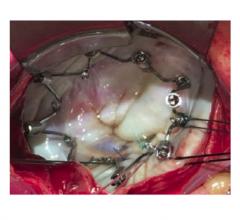
Boston Scientific Corp. announced it has closed an investment and entered into an acquisition option agreement with Millipede Inc., a privately-held company that has developed the IRIS Transcatheter Annuloplasty Ring System for the treatment of severe mitral regurgitation (MR). Under the terms of the agreements, Boston Scientific has purchased a portion of the outstanding shares of Millipede along with newly issued shares of the company for a total consideration of $90M. Boston Scientific has the option to acquire the remaining shares of the company at any time prior to the completion of a first in human clinical study that meets certain parameters. Upon the completion of the clinical study, Millipede has the option to compel Boston Scientific to acquire the remaining shares of the company. Each company's option period expires by the end of 2019. Completion of this acquisition would result in an additional $325M payment by Boston Scientific at closing with a further $125M becoming payable upon achievement of a commercial milestone.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
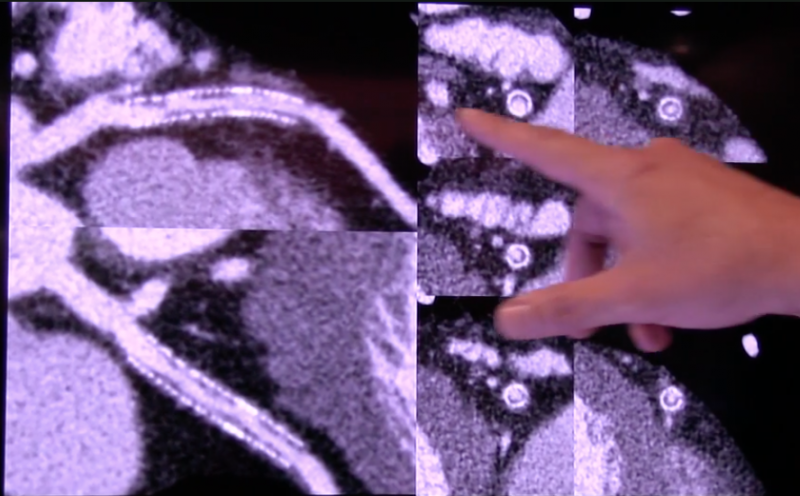
Medical imaging plays a key role in cardiology, and most of the newest radiology technology advances are first unveiled ...
January 23, 2018 – Imricor Medical Systems announced the completion of enrollment for the clinical study to evaluate ...
January 23, 2018 – Siemens Healthineers and Florida Hospital, part of Adventist Health System, have announced a multi ...
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
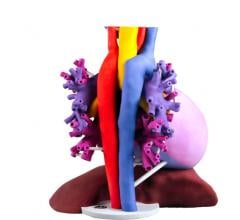
January 23, 2017 – At the annual meeting of Radiological Society of North America, 3D Systems announced a new D2P (DICOM ...
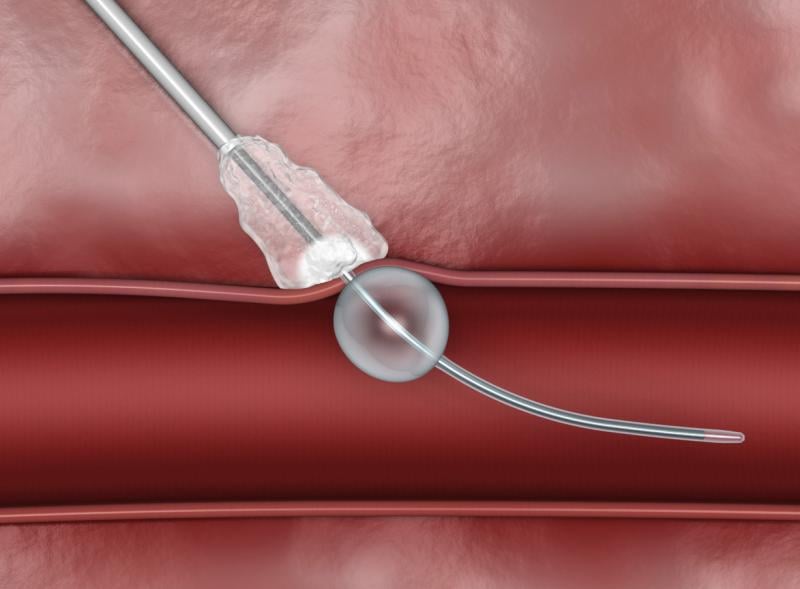
While manual compression remains the gold standard for hemostasis of catheterization vascular access site arteriotomies ...
The U.S. Food and Drug Administration (FDA) announced last week it is providing information and recommendations regarding the Zoll LifeVest 4000 due to concerns that the device may fail to deliver treatment to the patient if the device is not replaced soon after displaying "Call for service: Device has a problem that may require service. Call Zoll for service, Message Code 102." Failure to contact Zoll and immediately replace the device after Message Code 102 appears on the device screen may result in serious patient harm or death of the patient because the device may fail to deliver therapy appropriately when needed.
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...
January 22, 2018 — Baxter International Inc. announced the U.S. Food and Drug Administration (FDA) approval of ...
Ligand Pharmaceuticals Inc. announced initiation of a program to develop contrast agents with reduced renal toxicity. Through this new, internally-funded program, Ligand intends to advance products toward proof-of-concept, followed by selling or out-licensing them for further development and commercialization. The program will leverage Ligand’s patented Captisol technology, as well as data and intellectual property obtained through its acquisition of Verrow Pharmaceuticals, a privately-held Lenexa, Kansas-based medical invention company that Ligand acquired in January 2018 for $2 million in cash plus earnouts.
Abbott last week announced CE Mark approval for the company's new Advisor HD Grid Mapping Catheter, Sensor Enabled, a product designed to advance cardiac mapping during cardiac ablation to treat patients with complex cardiac arrhythmias. With the European launch of this latest addition to Abbott’s electrophysiology portfolio, the company is offering physicians a mapping catheter with what it calls a first-of-its-kind grid configuration of electrodes for improved data collection that supports the creation of high-density mapping of cardiac tissue to support optimal treatment for patients.

 January 25, 2018
January 25, 2018