The U.S. Food and Drug Administration (FDA) announced that International Laboratories LLC is voluntarily recalling Lot# 117099A of Clopidogrel Tablets, USP 75 mg, packaged in bottles of 30 tablets, to the consumer level due to mislabeling. The product is labeled as Clopidogrel Tablets USP, 75 mg but may contain Clopidogrel 75mg or Simvastatin Tablets USP 10 mg.
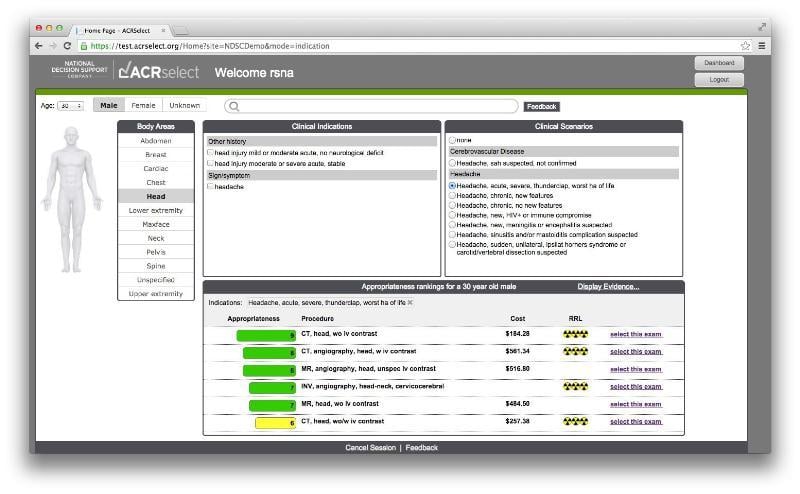
January 18, 2018 — Change Healthcare announced the acquisition of National Decision Support Company (NDSC), a leader in ...
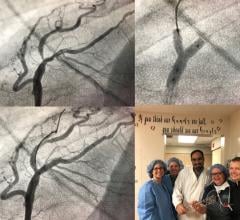
January 18, 2018 – West Hills Hospital & Medical Center, a full-service acute care facility, announces the first ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
January 18, 2018 – Johnson & Johnson Medical Devices Companies announced that Biosense Webster, Inc., a worldwide leader ...
Here is a list of what I think were some of the top interventional technologies discussed at the 2017 Transcatheter ...
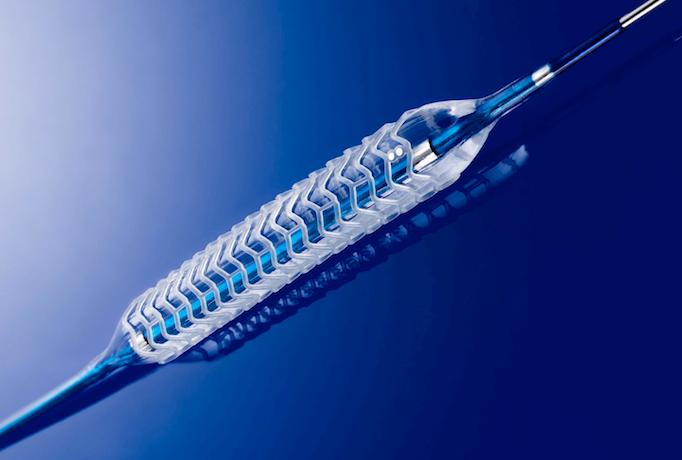
There was no shortage of interest in bioresorbable stent technologies at the many sessions offered on this topic or ...
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
Philips recently announced the launch of Technology Maximizer, a cross-modality program designed to boost the clinical capabilities and performance of imaging equipment through proactive upgrades. Technology Maximizer provides confidence to radiology and cardiology department leaders and clinicians that their systems are up to date and compliant at a fraction of the cost of individual upgrades, according to Philips.
The Society for Vascular Ultrasound (SVU), the Society for Vascular Surgery (SVS) and Medstreaming-M2S announced the development of the Vascular Quality Initiative (VQI) Vascular Ultrasound Registry. This Registry represents an expansion of the SVS VQI, which will combine noninvasive (vascular ultrasound) testing data with vascular treatment and outcomes data, making it possible to analyze the relationships between diagnosis and care provided to patients with vascular disease.
Secant Group, in partnership with its sister company SanaVita Medical, announced new technology to advance cardiovascular regenerative medicine with the development of a synthetic, small-bore vessel that encourages endogenous regeneration and new vessel formation. The technology is based on the company’s textile forming capabilities that can produce a hollow lumen construct infused with Secant’s proprietary Regenerez bioresorbable polymer technology. The new small-bore vessel supports the regeneration of new vascular tissue structures without the need for cell seeding or biologic growth promoters.
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
January 16, 2018 — Researchers from the NUST MISIS Engineering Center for Industrial Technologies in Russia have ...
Miracor Medical Systems GmbH (Miracor Austria) and Miracor Medical SA (Miracor Medical) announced the closing of €25m as part of a Series D financing round. The new capital will be used to further develop and commercialize the PiCSO Impulse System. The round was led by Ming Capital (Shenzhen, China) and co-led by a strategic investor.
American College of Cardiology (ACC) Chief Executive Officer Shalom “Shal” Jacobovitz will be leaving the College effective Feb. 2, 2018, to lead CiVi Biopharma Inc., a privately held biopharmaceutical company. Cathy Gates, ACC chief operating officer and executive vice president, will serve as interim CEO.
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...
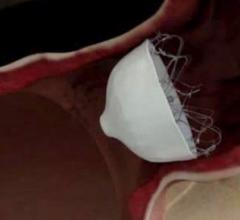
Medtronic plc announced the company’s Neurovascular business unit received U.S. Food and Drug Administration (FDA) clearance of the Riptide Aspiration System, adding a tool to its acute ischemic stroke (AIS) product portfolio.

The development of atrial fibrillation (AFib or AF) ablation technologies over the past 20 years has been a constant ...
It is not the amount of fat in your body but where it is stored that may increase your risk for heart attack, stroke and diabetes, according to a new study presented at the annual meeting of the Radiological Society of North America (RSNA), Nov. 26-Dec. 1 in Chicago. The study looked at the differences in fat distribution patterns among overweight and obese men and women and their associated cardiometabolic risk.

 January 19, 2018
January 19, 2018