October 4, 2023 — Anumana, Inc., a leading AI-driven health technology and nference portfolio company working in ...
October 4, 2023 — MedAxiom, a premier source for cardiovascular organizational performance solutions, has released its ...
October 4, 2023 — Butterfly Network, Inc. a digital health company transforming care through the power of portable ...
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
October 5, 2023 — A newly-issued "Scientific Statement on Durable Mechanical Circulatory Support" in the October issue ...
October 4, 2023 — The American College of Cardiology (ACC) recently announced that it has started a strategic innovation ...
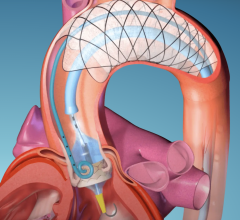
October 3, 2023 — EnCompass Technologies, Inc., a privately held medical device company focused on protecting patients ...
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
October 3, 2023 -- Boston Scientific launched the LUX-Dx II+ Insertable Cardiac Monitor (ICM) System, a next-generation ...
October 3, 2023 — Vascular surgeons Theodore Teruya, MD, and Sally Schonefeld, MD, have joined the Division of Vascular ...
October 3, 2023 — A discovery of a mutation in the gene ACTA2 has given researchers, led by Dianna Milewicz, MD, PhD ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
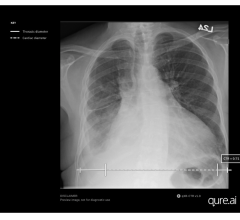
September 29, 2023 — Qure.ai, a leading global innovator in radiology AI solutions, has announced FDA clearance for ...
September 29, 2023 —Nano-X Imaging, an innovative medical imaging technology company, today announced that HealthCCSng ...
September 28, 2023 — Merck announced that the U.S. Food and Drug Administration (FDA) has accepted for priority review a ...
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
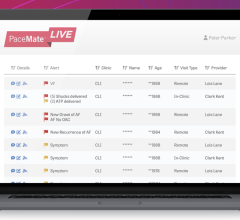
September 28, 2023 — PaceMate, a market leading cardiac data management platform, announced today industry partnership ...
September 28, 2023 — MarinHealth today announced that Pankaj Malhotra, MD, a non-invasive cardiologist with additional ...
September 27, 2023 — The healthcare landscape has undergone rapid changes in recent years, requiring clinicians and ...

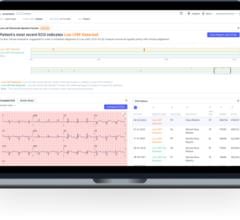
 October 04, 2023
October 04, 2023