September 11, 2017 — According to the latest market study released by Technavio, the global electrophysiology ...
Cardiac Resynchronization Therapy Devices (CRT)
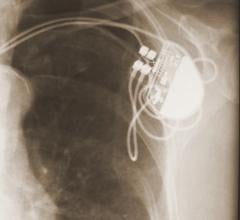
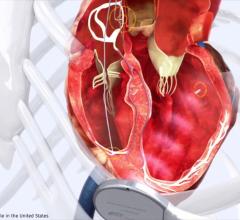
Cardiac Resynchronization Therapy Devices (CRT) are cardiac electrophysiology (EP) systems that include a small electronic device that is surgically implanted into a pocket of skin to help both ventricles contract together. They are also called biventricular pacemakers. These systems can be used for pacing only, or can include a built-in defibrillator.
August 21, 2017 — Biotronik announced U.S. Food and Drug Administration (FDA) approval and commercial availability of ...
July 21, 2017 — Biotronik announced U.S. Food and Drug Administration (FDA) approval and availability of the Intica DX ...
July 13, 2017 — Experts in heart failure management gathered in June to discuss varying scientific evidence in their ...
July 6, 2017 — New data showed use of Medtronic’s cardiac resynchronization therapy (CRT) devices with its proprietary ...
July 3, 2017 — Medtronic recently announced that its Reactive ATP therapy slows the progression of atrial fibrillation ...
Leyla Elif Sade, M.D., MESC, professor of cardiology at Başkent University, Ankara, Turkey, discusses use of echo for ...

Electrophysiology (EP) technology has been advancing rapidly the past few years with new ablation tools to improve ...
June 20 2017 — Pacemakers and other cardiac devices can help solve forensic cases, according to a study presented at the ...
DAIC Editor Dave Fornell takes a tour of some of the most innovative new electrophysiology (EP) technology at the 2017 H ...
Bruce Wilkoff, M.D., director of cardiac pacing and tachyarrhythmia devices at Cleveland Clinic, discusses advancements ...
This video, provided by ERB, demonstrates the function and implantation of the WiSE CRT (Wireless Stimulation ...
May 15, 2017 – The U.S. Food and Drug Administration (FDA) has granted market clearance Medtronic’s portfolio of ...
May 11, 2017 — Biotronik announced U.S. Food and Drug Administration (FDA) approval of the company's MultiPole Pacing ...
May 10, 2017 — Biotronik announced the availability of the first U.S. Food and Drug Administration (FDA)-approved cardi ...

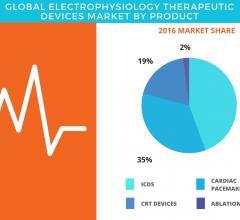
 September 11, 2017
September 11, 2017