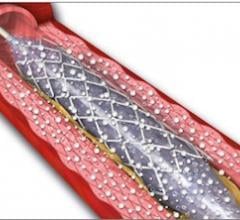
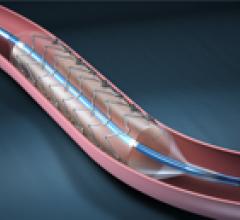
November 1, 2012 — It looks as though drug-eluting balloons (DEBs), already widely used in Europe, are set to burst into ...
Drug-Eluting Balloons
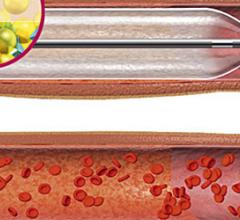
This channel includes news and new technology innovations for drug-coated balloons (DCB), also referred to as drug-eluting balloons. These are used to treat peripheral and coronary artery lesions and restenosis. The balloons carry an antiproliferative drug that is delivered to the wall of arteries when the balloon is expanded. The drug helps prevent neointimal hyperplasia (scar tissue growth) caused by trauma when the vessel segment is treated for atherosclerotic lesions with balloon angioplasty. DCBs can be used to treat hyperplasia in arteriovenous (AV) access fistulae in dialysis patients, where the vessel undergoes repeated trauma from regular punctures. DCBs also are used to treat in-stent restenosis due to scar tissue proliferation inside stents, which can cause a vessel to occlude.
September 21, 2012 — Cardionovum GmbH announced that the results of a preclinical study and a first-in-man clinical ...
July 27, 2012 — C. R. Bard Inc. announced that its Lutonix technology center has completed patient enrollment into its ...
July 9, 2012 — Cardionovum GmbH announced it has initiated in vivo testing of its second drug-eluting balloon (DEB) Rest ...
June 13, 2012 — Physicians presented at EuroPCR 2012 the results of two multicenter, randomized controlled trials, the ...
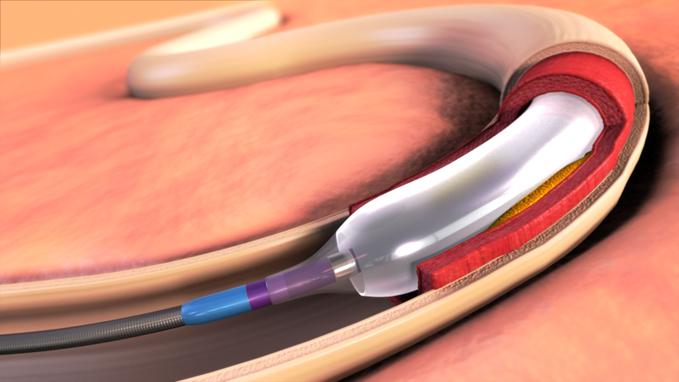
April 17, 2012 –– Expanding its commitment to developing innovative treatments for cardiovascular disease and the ...
March 20, 2012 - MarBlue Medical announced the global product launch of its CE-marked drug-eluting balloon (DEB) Protege ...
January 25, 2012 — Physicians at University Hospitals (UH) Case Medical Center enrolled their first patient in LEVANT 2 ...
January 6, 2012 - C. R. Bard recently announced it acquired drug-eluting balloon maker Lutonix Inc. for $225 million ...
December 28, 2011 – Good Samaritan Hospital in Los Angeles is participating in the Levant 2 clinical trial to ...
November 14, 2011 — A clinical trial of patients with restenosis in drug-eluting stents (DES) in native coronary ...
November 14, 2011 – Medrad Inc. announced that five-year data from the THUNDER trial [1] demonstrated a 59 percent ...
September 15, 2011 – The Cardiovascular Research Foundation (CRF) has announced the late breaking trials and first ...
September 15, 2011 — Very positive results were reported in the first account of a clinical study evaluating the ...
July 26, 2011 – The first patient has been enrolled in the LEVANT 2 clinical trial, a global, multi-center, randomized ...

 November 01, 2012
November 01, 2012