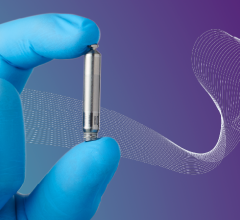
April 12, 2022 — Fitbit received clearance from the U.S. Food and Drug Administration for its new PPG (photoplethysmogra ...
EP Lab
This channel includes news and new technology innovations for cardiac electrophysiology (EP) systems, techniques and devices using in EP labs. This includes implantable EP devices, pacemakers, implantable cardioverter defibrillators (ICD), cardiac resychronization therapy (CRT), ablation technologies, left atrial appendage (LAA) occlusion, atrial fibrilation (AF) and Holter monitors.
April 6, 2022 – Final results from the BIO|GUARD-MI study show a 31 percent reduction of MACE in a sub-group analysis of ...
April 5, 2022 — Abbott announced that the U.S. Food and Drug Administration (FDA) has approved the Aveir single-chamber ...
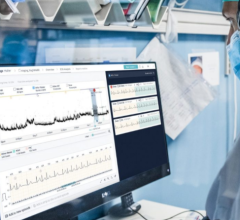
When the patients of Michael Boler, M.D. need cardiac monitoring, the Holter monitor is no longer his first choice. “The ...
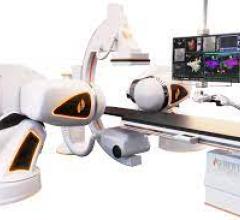
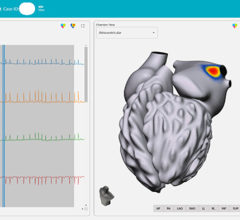
April 1, 2022 – Stereotaxis, the global leader in innovative robotic technologies for the treatment of cardiac ...
April 1, 2022 — Cardiologs announced a poster presentation at the 2022 American College of Cardiology (ACC) meeting in ...
March 31, 2022 — The annual congress of the European Heart Rhythm Association (EHRA), a branch of the ESC, will be held ...
Apple created a stir when it announced in 2018 that its Apple Watch Series 4 was the first consumer health and fitness ...
March 29, 2022 – Eko, a digital health company advancing heart and lung disease detection, has announced the launch of ...
March 14, 2022 – Stroke is an acute disease of the brain and the most common cause of permanent disability in adults in ...
March 13, 2022 – Philips has announced new research evaluating mobile cardiac outpatient telemetry (MCOT) as a first ...
Washington Health System (WHS) provides healthcare services at more than 40 offsite locations across three counties in ...
March 13, 2022 – People with a congenital heart defect who were hospitalized with COVID-19 infection were at higher risk ...
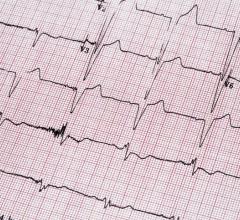
March 9, 2022 – Point-of-care screening for atrial fibrillation (A-fib) using a new generation of handheld electrocardio ...
March 3, 2022 – CIRCA Scientific, Inc. has announced that its S-CATH M Esophageal Temperature Probe is now cleared for ...
Apple created a stir when it announced in 2018 that its Apple Watch Series 4 was the first consumer health and fitness ...
February 28, 2022 – iRhythm Technologies, Inc., a leading digital healthcare solutions company focused on the ...
February 25, 2022 – Abbott has announced that the U.S. Food and Drug Administration (FDA) has approved an expanded ...
February 16, 2022 – A new way of treating arrhythmias, including atrial fibrillation (AF) — the most common heart ...

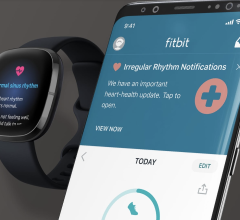
 April 12, 2022
April 12, 2022