July 31, 2024 — BioCardia, Inc., a developer of cellular and cell-derived therapeutics for the treatment of ...
EP Lab
This channel includes news and new technology innovations for cardiac electrophysiology (EP) systems, techniques and devices using in EP labs. This includes implantable EP devices, pacemakers, implantable cardioverter defibrillators (ICD), cardiac resychronization therapy (CRT), ablation technologies, left atrial appendage (LAA) occlusion, atrial fibrilation (AF) and Holter monitors.
July 31, 2024 — A novel study co-authored by a heart failure cardiologist at University Hospitals Harrington Heart & ...
July 24, 2024 — During the American Heart Association Basic Cardiovascular Sciences Scientific Sessions 2024, BCVS ...
When the patients of Michael Boler, M.D. need cardiac monitoring, the Holter monitor is no longer his first choice. “The ...
July 24, 2024 — Volta Medical, a health technology company developing artificial intelligence (AI) solutions to assist ...
July 17, 2024 — BioCardia, Inc., a company focused on cellular and cell-derived therapeutics for the treatment of ...
July 16, 2024 — Ultrahuman, a pioneer in wearable technology, launches PowerPlugs, a platform for individual apps and ...
Apple created a stir when it announced in 2018 that its Apple Watch Series 4 was the first consumer health and fitness ...
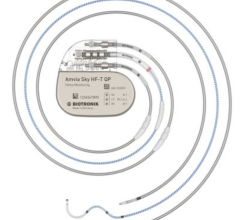
July 11, 2024 — Dr. Fadi Mansour performed the first Canadian implant of BIOTRONIK’s newest pacemaker and CRT-P ...

In a room of 20 people, it’s likely that about 10 of them, or half, will presently have some form of cardiovascular ...
July 8, 2024 — Pulsed field ablation (PFA) is safe for treating patients with common types of atrial fibrillation (AF) ...
Washington Health System (WHS) provides healthcare services at more than 40 offsite locations across three counties in ...
June 25, 2024 — Heart Hospital of New Mexico at Lovelace Medical Center (HHNM) and GE HealthCare (announced HHNM as the ...
June 21, 2024 — Cardiomatics, a pioneer in AI-powered ECG analysis solutions, and the Biotronik sales organization for ...
June 20, 2024 — A programming algorithm, being tested by HonorHealth Research Institute for those patients with new or ...
Apple created a stir when it announced in 2018 that its Apple Watch Series 4 was the first consumer health and fitness ...
June 19, 2024 — When electrophysiologist Eugenio Cingolani, MD, isn’t seeing patients, he can usually be found in his ...

While the spotlight in 2023 shone on developments around Generative Artificial Intelligence (AI), away from the ...
June 17, 2024 — Elutia Inc., a pioneer in drug-eluting biomatrix products, today announced that its Antibiotic-Eluting ...


 July 31, 2024
July 31, 2024