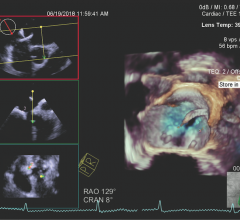
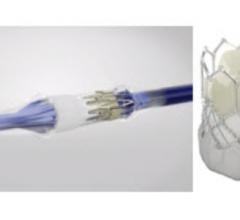
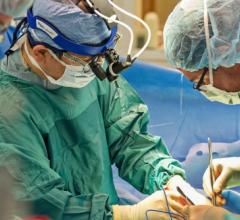
This 360 degree view shows staff at the University of Colorado Heart and Vascular Center performing live transesophageal ...
Heart Valve Technology
This channel includes news and new device innovations about heart valve technologies, including the aortic valve, mitral valve, pulmonic valve, and tricuspid valve. This includes information on transcatheter valve technologies like transcatheter aortic valve replacement (TAVR, or implantation TAVI), transcatheter mitral valve repair or replacement (TMVR), transcatheter and surgical valve repairs, and surgical replacement valves. Newer devices are now being used for transcatheter tricuspid valve repair replacement (TTVR).
A discussion with Ruth Fisher, MBA, vice president of the Henry Ford Hospital structural heart program, and Janet Wyman ...
Marvin Eng, M.D., structural fellowship director at Henry Ford Health System, and William O'Neill, M.D., director of the ...
A discussion with William O’Neill, M.D., director of the Henry Ford structural heart program, Ruth Fisher, MBA, vice ...
September 24, 2019 – Medtronic announced U.S. Food and Drug Administration (FDA) approval and U.S. launch of the Evolut ...
September 20, 2019 – Abbott announced approvals in Europe for two of its pediatric devices — the Masters HP 15mm ...
September 20, 2019 — BioTrace Medical Inc. announced the company’s key activities at the 31st annual Transcatheter ...
September 11, 2019 — Percutaneous reduction of secondary mitral regurgitation in patients with heart failure does not ...
September 5, 2019 — Abbott announced the launch of the company's TRILUMINATE Pivotal trial evaluating the safety and ...

September 3, 2019 — Here is the list of the most popular content on the Diagnostic and Interventional Cardiology (DAIC) ...
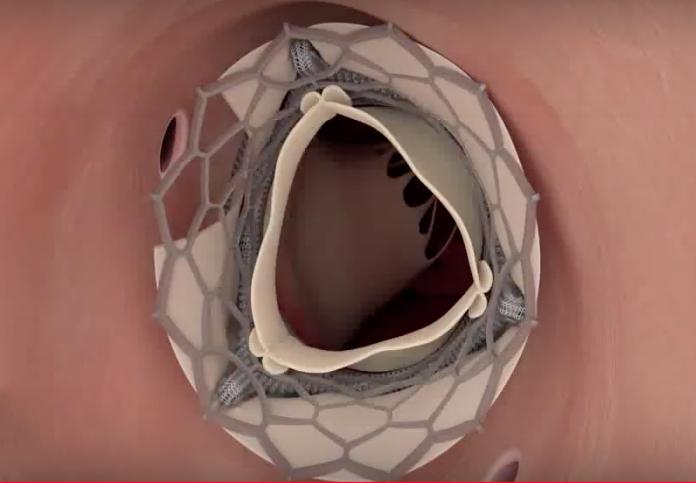
A novel technique has proven successful in preventing coronary artery obstruction during transcatheter aortic valve ...
John Carroll, M.D., FACC, FSCAI, director of interventional cardiology at the Cardiac and Vascular Center at the ...
August 22, 2019 — Edwards Lifesciences is recalling the Sapien 3 Ultra delivery system after receiving reports of burst ...
August 20, 2019 — Left ventricular assist devices (LVADs) have been shown to help leaky mitral valves that create ...
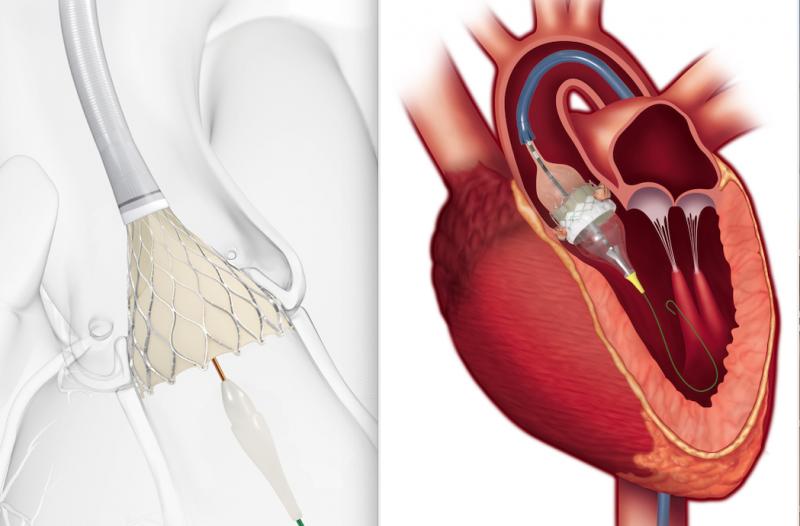
August 16, 2019 — In one coordinated move, the U.S. Food and Drug Administration (FDA) opened use of transcatheter ...

 October 02, 2019
October 02, 2019