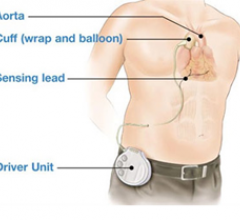
Two key news items in September made me think about the future direction of hemodynamic support, and that intra-aortic ...
Intra-Aortic Balloon Pumps (IABP)
This channel includes news and new technology innovations for intra-aortic balloon pumps (IABP). IABPs are a type of hemodynamic support device that is inserted into the aorta to help augment the flow of blood with a pulsing balloon that pushes the blood volume out of the the aorta to help perfuse organs and tissues.
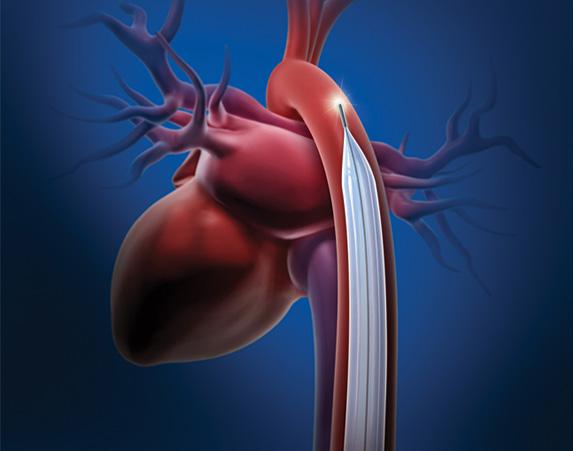
November 8, 2012 — A U.S. Food and Drug Administration (FDA) advisory committee is meeting Dec. 5 to discuss how to ...
October 30, 2012 — Maquet Cardiovascular received 510(k) clearance from the U.S. Food and Drug Administration (FDA) and ...

September 11, 2012 — Primary results from an ongoing trial by Maquet Cardiovascular using intra-aortic balloon pumps ...
September 10, 2012 — Maquet Cardiovascular LLC announced 30-day results from the large, randomized, multicenter intra ...
May 16, 2012 — Maquet Cardiovascular LLC announced this week that it has received 510(k) clearance from the U.S. Food ...
March 26, 2012 — Maquet announced positive long-term mortality results from the Balloon Pump-Assisted Coronary ...
Teleflex Inc. announced a new agreement with HealthTrust Purchasing Group L.P. (HealthTrust), for its Arrow intra-aortic ...
November 11, 2011 — Maquet Cardiovascular announced U.S. Food and Drug Administration (FDA) 510(k) clearance and CE mark ...
October 17, 2011 — Maquet Cardiovascular announced it has signed a three-year, dual-source contract with Premier ...
October 3, 2011 – Maquet Cardiovascular has signed a definitive agreement to acquire Atrium Medical Corp. for $680 ...
September 20, 2011 — Maquet Cardiovascular announced it has received both 510(k) clearance from the U.S. Food and Drug ...
August 29, 2011 — Recent data suggest post-operative outcomes of severe heart failure patients bridged short-term ...
June 15, 2011 – The U.S. Food and Drug Administration (FDA) said Maquet Datascope Corp. has issued a class I recall for ...
June 14, 2011 – An initial animal study of a next-generation cardiac assist device for heart failure patients using a ...


 December 07, 2012
December 07, 2012