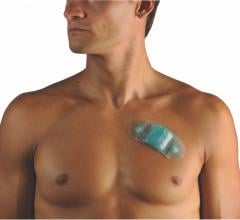
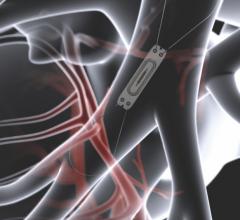
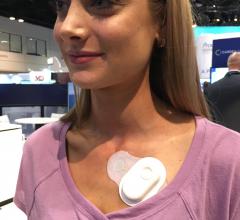
August 30, 2018 — At the 2018 European Society of Cardiology (ESC) Congress, cardiovascular technology startup Maisense ...
Patient Monitors
August 24, 2018 — U.S. Expeditions and Explorations (USX) and Cardiac Insight Inc. announced the successful completion ...
August 16, 2018 — Lexington Biosciences Inc. recently announced the completion of the initial HeartSentry study conducte ...
When the patients of Michael Boler, M.D. need cardiac monitoring, the Holter monitor is no longer his first choice. “The ...
August 15, 2018 — iBeat recently announced the release and shipping of its iBeat Heart Watch. The heart and blood flow ...
August 10, 2018 — Caretaker Medical, creator of the Caretaker4 wireless CNIBP Vital signs monitor for continuous non ...
July 23, 2018 — The U.S. Food and Drug Administration (FDA) has granted 510(k) clearance for the PocketECG Cardiac ...
Apple created a stir when it announced in 2018 that its Apple Watch Series 4 was the first consumer health and fitness ...
July 5, 2018 — Philips and Florida’s Jackson Health System, one of the nation's largest public health systems, announced ...
New patient monitoring technologies with wireless connectivity have enabled a revolution in cardiac event and Holter ...
May 17, 2018 — A new study published in JAMA Cardiology used the Zio continuous cardiac monitoring system by iRhythm to ...
Apple created a stir when it announced in 2018 that its Apple Watch Series 4 was the first consumer health and fitness ...
May 16, 2018 — Itamar Medical Ltd. announced the launch of SleePath, the first integrated e-health sleep apnea care ...
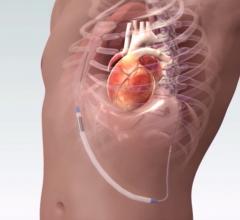
March 29, 2018 — Abbott announced the company has initiated the landmark GUIDE-HF clinical trial using the CardioMEMS HF ...
The show floors at the American College of Cardiology (ACC) 2018 meeting in March and at the Heart Rhythm Society ...
When the patients of Michael Boler, M.D. need cardiac monitoring, the Holter monitor is no longer his first choice. “The newer ECG devices we have now are so much less cumbersome. It’s like wearing a Band-Aid versus carrying a bulky device,” said the Greenwood, Mississippi internist. “My patients prefer the more comfortable, wire-free form factor, and the quality is as good as, or better, than the Holter,” continued Boler. “Plus, my patient compliance has increased. With the Holter, the leads sometimes come off. The patient may think the device isn’t working, so they take it off and we have to restart the process.”
March 9, 2018 — Philips recently announced the integration of its IntelliVue Guardian with automated Early Warning ...
February 27, 2018 — Medical devices, including cardiovascular implantable electronic devices, could be at risk for ...
February 20, 2018 – Lexington Biosciences, Inc., a development-stage medical device company, announced the commencement ...

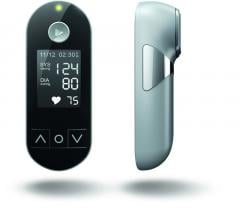
 August 30, 2018
August 30, 2018