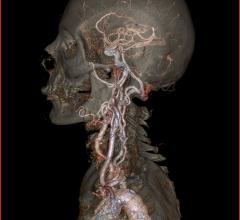
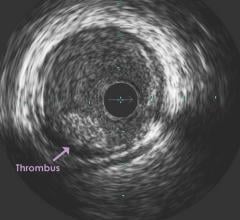
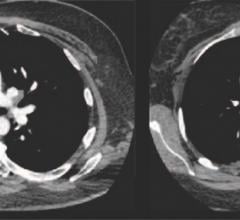
November 1, 2018 — A vaccine may one day be able to replace oral blood thinners to reduce the risk of secondary strokes ...
Pharmaceuticals
This channel includes news and new technology innovations for cardiovascular pharmaceutics. This includes antiplatelet agents, anticoagulation drugs, INR testing, NOAC, OAC, IV administered drugs such as Heprin, and dual antiplatelet therapy.
October 31, 2018 — Amgen announced a new Repatha cardiovascular outcomes study (FOURIER) analysis evaluating the effects ...
October 31, 2018 — Recent statin and medication advances have led some researchers to suggest surgical treatments for ...
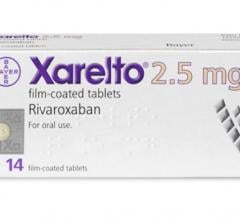
October 17, 2018 — The U.S. Food and Drug Administration (FDA) has cleared an additional indication for rivaroxaban ...
October 11, 2018 — Novel results from the landmark EMPA-REG OUTCOME trial suggest that treatment with Jardiance ...
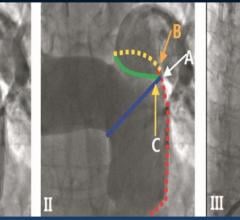
October 4, 2018 — Pulmonary artery denervation (PADN) is associated with significant improvements in hemodynamic and ...
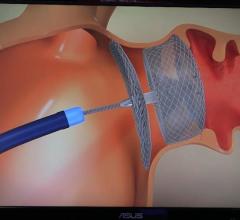
Ashish Pershad, M.D., medical director, structural heart program, Banner University Medical Heart Institute, Phoenix ...
September 7, 2018 — The antithrombin drug rivaroxaban does not reduce the risk of a composite endpoint of survival ...
September 7, 2018 — A new Phase 2 clinical trial looks to confirm the efficacy and safety of Thrombolytic Science LLC’s ...
September 6, 2018 — Tafamidis is the first treatment to improve survival and reduce hospitalizations in a rare heart ...
September 5, 2018 — Aspirin prevented serious vascular events in patients with diabetes who did not already have ...
September 4, 2018 — The jury is still out on whether people at moderate risk of a first heart attack or stroke should ...
August 31, 2018 — Use of an oral anticoagulant in medically ill patients for 45 days following hospital discharge ...
August 27, 2018 — Blood pressure and cholesterol-lowering drugs continue to improve survival in patients with hypertensi ...
June 29, 2018 — Results from the MERCURY PE study showed that low-risk pulmonary embolism (PE) patients treated with Xar ...

 November 01, 2018
November 01, 2018