September 20, 2013— The Mount Sinai Medical Center is participating in the nationwide Absorb III clinical trial testing ...
Stents Bioresorbable
This channel includes news and new technology innovations for bioresorbable stents (BRS). These devices are also referred to as bioabsorbable stents, bioresorbable scaffolds and dissolving stents. BRS are designed as an alternative to permanent metallic stent implants, which cause issues in a small number of patients with in-stent restenosis, late-stent thrombosis and require use of long-term antiplatelet therapy. Metallic stents also cause issues with CT and MRI imaging and may prevent future options for coronary bypass graft (CABG) surgery. BRS are supposed to remove avoid these issues by dissolving and disappearing from the vessel after a period of 2-4 years. This, returns the vessel to its natural state and allows for the return of vasodilatation and vasoconstriction. BRS have had some issues in clinical trials not being able to match the performance of standard metallic drug eluting stents (DES) because of their thick stent struts. Newer generation BRS are in development with struts smaller than 100 micros, with will be closer to those of current generation metallic stents
July 12, 2013 — Micell Technologies Inc. received CE mark approval for its MiStent sirolimus-eluting absorbable polymer ...
June 24, 2013 — Final six and 12-month results of the DIRECT first-in-man clinical study were presented by study ...
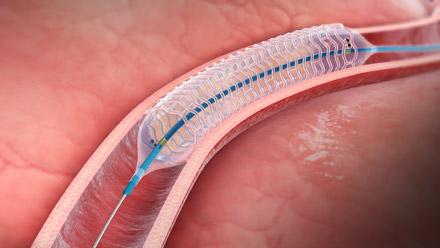
May 16, 2013 — Elixir Medical Corp. announced it received CE (Conformité Européenne) mark approval for its DESolve ...
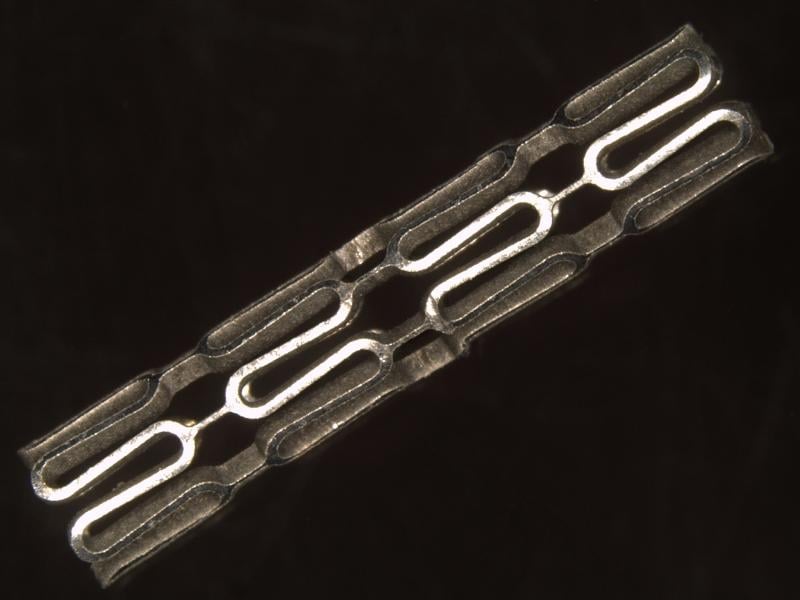
May 15, 2013 — In 2012, more than 3 million people had stents inserted in their coronary arteries. After about six ...
April 2, 2013 — Reva Medical Inc. announced that it has initiated patient enrollment into a clinical trial with its ...
March 20, 2013 — Svelte Medical Systems announced treatment of the first patient in the DIRECT II (Direct Implantation ...
March 15, 2013 — Abbott announced positive long-term results for the company's innovative Absorb Bioresorbable Vascular ...
The interventional cardiology market includes, but is not limited to, drug-eluting stents, bare-metal stents ...
January 30, 2013 — Coronary artery disease and peripheral artery disease are global health issues that affect millions ...
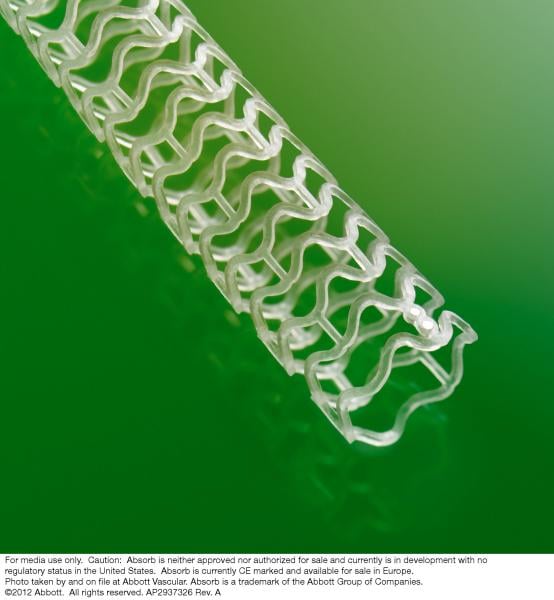
January 8, 2013 — Abbott announced the initiation of the ABSORB III clinical trial in patients in the United States. The ...
November 7, 2012 — Micell Technologies Inc. announced positive data from two clinical studies of its investigational ...

October 31, 2012 — Boston Scientific Corp. received CE mark approval for the Synergy everolimus-eluting platinum ...

October 30, 2012 — Elixir Medical Corp. said enrollment is complete in its 120-patient, pivotal clinical trial ...

October 19, 2012 — The 24th Transcatheter Cardiovascular Therapeutics (TCT) conference, sponsored by the Cardiovascular ...


 September 20, 2013
September 20, 2013


