Stents Drug Eluting
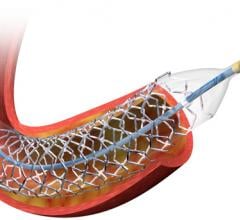
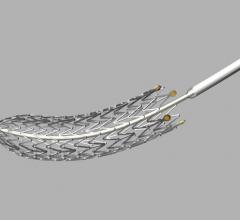
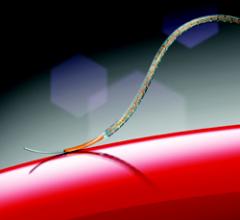
This channel includes news and new technology innovations for drug eluting stents (DES). These drug coated stents were developed to solve a frequent problem with bare metal stents, which can cause neointimal hyperplasia (scar tissue growth) in some patients. The antiproliferative drugs used on DES prevent the growth of tissue. One downside of DES is the requirement for patients to take long-term antiplatelet therapy to prevent the possible formation of clots on these stents. Newer generation DES use technologies help the vessels heal faster, which may allow reduce the duration of dual antiplatelet therapy (DAPT), or use a single drug, usually eliminating aspirin. This section includes news for both metallic and bioresorbable drug-eluting stents and related clinical trial data.
September 27, 2013 — Patient enrollment has been initiated in a post-market registry for the COMBO Dual Therapy Stent to ...
September 17, 2013 — In a significant milestone toward obtaining additional key regulatory approvals for the Synergy dru ...
August 19, 2013 — Cook Medical is again shipping its Zilver PTX drug-eluting peripheral stent to medical centers in the ...
August 16, 2013 — Patient enrollment has been initiated in a post-market registry for the Combo Dual Therapy Stent to ...
July 1, 2013 — Primary end-point results from the BIOFLOW-II clinical study demonstrating the non-inferiority of the ...
June 24, 2013 — Final six and 12-month results of the DIRECT first-in-man clinical study were presented by study ...
June 11, 2013 — New long-term data from the DIVERGE clinical study, presented at EuroPCR 2013 by Principal Investigator ...
May 28, 2013 — Abbott announced CE mark in Europe for the Xience Xpedition 48 Everolimus Eluting Coronary Stent System ...
April 30, 2013 — Cook Medical has initiated a global voluntary recall of its Zilver PTX Drug Eluting Peripheral Stent ba ...
March 28, 2013 — Cardiologists at ACC.13 learned that patients with coronary artery disease who received a Resolute drug ...
March 20, 2013 — Svelte Medical Systems announced treatment of the first patient in the DIRECT II (Direct Implantation ...

 November 01, 2013
November 01, 2013