ACC together with AHA and other key healthcare organizations, has launched "Door to Balloon (D2B): An Alliance ...
Stents Drug Eluting
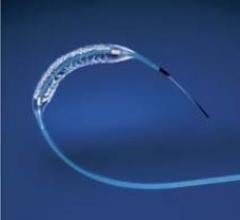
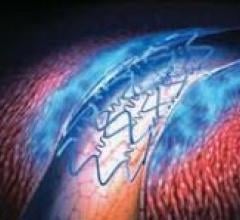
This channel includes news and new technology innovations for drug eluting stents (DES). These drug coated stents were developed to solve a frequent problem with bare metal stents, which can cause neointimal hyperplasia (scar tissue growth) in some patients. The antiproliferative drugs used on DES prevent the growth of tissue. One downside of DES is the requirement for patients to take long-term antiplatelet therapy to prevent the possible formation of clots on these stents. Newer generation DES use technologies help the vessels heal faster, which may allow reduce the duration of dual antiplatelet therapy (DAPT), or use a single drug, usually eliminating aspirin. This section includes news for both metallic and bioresorbable drug-eluting stents and related clinical trial data.
Results from the Danish SORT-OUT II study, the largest randomized trial to date comparing Taxus and Cypher drug-eluting ...
The Certification Commission for Healthcare Information Technology is inviting organizations to submit their ambulatory ...
A subset analysis of diabetic patients in the SPIRIT II Clinical trial of the XIENCE V Everolimus Eluting Stent System ...
Early clinical results from Abbott's ongoing ABSORB clinical trial, the world's first study to evaluate the ...
Boston Scientific has European CE clearance to begin marketing a private-label XIENCE V everolimus-eluting coronary ...
An FDA-appointed advisory panel to review drug-eluting stents could have detrimental effects on the $6 billion-a-year ...
Abbott Vascular says it will begin launching its XIENCE V stent system immediately in most European countries, focusing ...
Cordis Corp. has launched the CYPHER SELECT Plus Stent, the first third-generation DES, in Western and Eastern Europe ...
Positive six-month results of Abbott's SPIRIT II clinical trial of the XIENCE V Everolimus Eluting Coronary Stent ...
The FDA granted Conor Medsystems Inc. full approval to enroll up to 1,700 patients in its heart stent clinical ...
The TAXUS Express2 is a stainless steel stent that uses Paclitaxel and Translute polymer to control the drug release ...
Using a closed-cell design, the CYPHER stent delivers sirolimus, a drug that selectively targets and interrupts ...
The FDA has approved revised labeling for the instructions for use of the CYPHER Sirolimus-eluting Coronary Stent ...
Are drug-eluting stents destined to fail?
In Part 1 of this investigation, the connection of DES and thrombogenicity ...

 November 12, 2006
November 12, 2006