Edwards Lifesciences Corp. produces the Carpentier-Edwards
The PERIMOUNT Magna mitral valve was launched in ...
This structural heart channel includes news, videos, podcasts and other content related to diagnosis and treatment of structural heart disease. Topics covered include heart valve repair and replacement, transcatheter aortic valve replacement (TAVR), transcatheter mitral valve replacement (TMVR), transcatheter tricuspid valve replacement (TTVR), left atrial appendage (LAA) occlusion, heart failure interventional device therapies, and closing holes in the heart using, including occlusion of atrial septal defects (ASDs), ventricular septal defects (VSDs) and patent foramen ovales (PFOs).
Edwards Lifesciences Corp. produces the Carpentier-Edwards
The PERIMOUNT Magna mitral valve was launched in ...
September 3, 2008 - Edwards Lifesciences Corp. today said it received FDA approval for the Carpentier-Edwards PERIMOUNT ...
August 28, 2008 - CoreValve said today an Australia/New Zealand-focused clinical evaluation of its proprietary ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...
August 18, 2008 - Atritech Inc. today said it filed its pre-market approval application (PMA) with the FDA for its ...
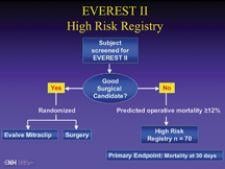
Preliminary results of a multicenter study show that the use of tiny clips to repair the mitral valve may someday ...
June 25, 2008 - Health Canada - Medical Device Bureau, Therapeutic Products Programme, has approved the use of the ATS ...
June 25, 2008 - A new cardiac treatment facility that couples the benefits of interventional cardiology with ...
June 16, 2008 – Toshiba America Medical Systems’ Infinix Hybrid cardiovascular X-ray imaging system will be featured in ...
May 13, 2008 - Edwards Lifesciences Corp. launched a next-generation transfemoral delivery system for the Edwards SAPIEN ...
April 22, 2008 - A new type of medication based on raising high-density lipoproteins (HDL), can lead to an ...
April 21, 2008 - The first patients were treated in a U.S. feasibility study using the Edwards Lifesciences SAPIEN ...
April 14 - Doctors implanted the ATS 3f Aortic Bioprosthesis, marking the first North American commercial implant after ...
April 14, 2008 - ESTECH will showcase its new CG LiV Kits, designed to enable mitral and aortic valve procedures ...
March 24, 2008 – Doctors implanted the first CryoValve SG pulmonary human heart valve in a patient at St. Louis Children ...
The Society for Cardiovascular Angiography and Interventions (SCAI) has partnered with the American College of ...