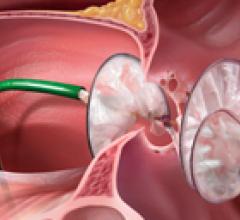
March 5, 2008 - Edwards Lifesciences Corp. reported that the first three human implants of the next-generation ...
Structural Heart
This structural heart channel includes news, videos, podcasts and other content related to diagnosis and treatment of structural heart disease. Topics covered include heart valve repair and replacement, transcatheter aortic valve replacement (TAVR), transcatheter mitral valve replacement (TMVR), transcatheter tricuspid valve replacement (TTVR), left atrial appendage (LAA) occlusion, heart failure interventional device therapies, and closing holes in the heart using, including occlusion of atrial septal defects (ASDs), ventricular septal defects (VSDs) and patent foramen ovales (PFOs).
W. L. Gore & Associates (Gore) announced that the FDA granted approval for the use of GORE HELEX Septal Occluder ...
February 11, 2008 - Leman Cardiovascular’s animal studies to evaluate the performance in both the aortic and mitral ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...
February 11, 2008 – A new minimally invasive vascular occlusion device designed to close a patent ductus ...
February 8, 2008 - CryoLife Inc. received 510(k) clearance from the FDA for its CryoValve SG pulmonary human heart ...
CryoLife Inc.'s CryoValve SG pulmonary human heart valve is processed with the company’s proprietary SynerGraft ...
February 5, 2008 – A medical team led by Adrian Ebner, M.D., of French Hospital and Lutz Buellesfeld, M.D. of Helios ...
January 30, 2008 - Aporo Biomedical signed an exclusive global licensing agreement with mNEMOSCIENCE GmbH for its ...
January 29, 2008 - Edwards Lifesciences Corp. received conditional approval from the FDA for the addition of the ...
January 28, 2008 - Arbor Surgical Technologies Inc. closed a $20 million dollar Series C financing round led by ...
January 22, 3008 - Cardica Inc. and Intuitive Surgical Inc. will host an evening symposium, demonstrating how surgeons ...
January 7, 2008 - Cardica Inc. received nearly $2 million in payments from Cook Medical (Cook) for reaching ...
January 3, 2008 - Medical Simulation Corp. and Edwards Lifesciences Corp. will launch the Edwards SAPIEN Transcatheter ...
December 18, 2007 - ATS Medical Inc. today announced it has received FDA clearance for the ATS Open Pivot AP360 ...
December 11, 2007 - ValveXchange, developer of a next-generation replacement heart valve technology, won the third ...

 March 04, 2008
March 04, 2008