September 2, 2022 — Acetazolamide added to intravenous loop diuretics decreases congestion within three days in patients with acute decompensated heart failure, according to late breaking research presented in a Hot Line session today at ESC Congress 2022.1
Principal investigator Professor Wilfried Mullens of Hospital Oost-Limburg (ZOL), Genk, Belgium said: “ADVOR was the largest randomized diuretic trial ever performed in patients with acute decompensated heart failure. Acetazolamide is easy to use, safe, effective, off-patent and cheap. It is therefore expected that the results of ADVOR will lead to a paradigm shift in the way physicians worldwide treat acute decompensated heart failure.”
Acute heart failure is a life-threatening condition requiring urgent evaluation and treatment. It is usually due to an acute deterioration of chronic heart failure. Acute decompensated heart failure is the most common form, accounting for up to 70% of presentations.2 Guidelines recommend intravenous loop diuretics to improve symptoms of fluid overload, but many patients still have residual congestion3 which is a strong predictor of poor outcome. Sequential diuretic therapy has been suggested as a more effective decongestive strategy.2,4 However, decisive evidence is lacking on the optimal drugs, dosing schedule, and route of administration.
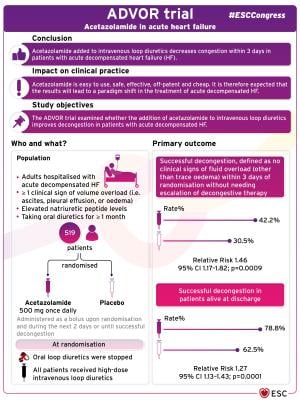
Acetazolamide is a carbonic anhydrase inhibitor that reduces proximal tubular sodium reabsorption. The ADVOR trial examined whether the addition of acetazolamide to intravenous loop diuretics improves decongestion in patients with acute decompensated heart failure.5
The trial enrolled 519 adults hospitalized with acute decompensated heart failure at 27 centers in Belgium. The average age was 78 years and 63% were men. Patients had at least one clinical sign of volume overload (i.e. ascites, pleural effusion, or oedema), elevated natriuretic peptide levels, and had been taking oral diuretics for at least one month. Patients were randomized (1:1) to intravenous acetazolamide (500 mg once daily) or placebo, administered as a bolus upon randomization and during the next two days or until successful decongestion. At randomization, oral loop diuretics were stopped and all patients received high-dose intravenous loop diuretics.
The primary endpoint was successful decongestion, defined as no clinical signs of fluid overload (other than trace oedema) within three days of randomization without needing escalation of decongestive therapy. The primary outcome occurred in 108 of 256 patients (42.2%) in the acetazolamide group and 79 of 259 patients (30.5%) in the placebo group, for a relative risk (RR) of 1.46 (95% confidence interval [CI] 1.17–1.82; p=0.0009). Of those alive at discharge, 190 of 241 in the acetazolamide group (78.8%) and 145 of 232 in the placebo group (62.5%) had successful decongestion (RR 1.27; 95% CI 1.13–1.43; p=0.0001).
Regarding key secondary endpoints, patients in the acetazolamide group had a shorter hospital stay (an average of 8.8 days) compared with those in the placebo group (average of 9.9 days; p=0.02) but there was no difference between groups in the composite outcome of all-cause mortality and hospitalization for heart failure within three months.
Professor Mullens said: “Patients treated with acetazolamide had more diuresis and natriuresis, and were more likely to be discharged without residual signs of volume overload. There did not appear to be an increase in adverse events with the drug. Participants had similar characteristics to patients in real world practice, including a high degree of congestion, advanced age and many comorbidities.”
For more information: www.escardio.org
References and Notes
1ADVOR will be discussed during:
Hot Line Session 2 on Saturday 27 August at 08:30 to 10:00 CEST in the Barcelona auditorium.
Meet the Trialist – ADVOR on Saturday 27 August at 11:30 to 11:50 CEST on the ESC TV Stage.
2McDonagh TA, Metra M, Adamo M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42:3599–3726.
3Felkner GM, Lee KL, Bull DA, et al. Diuretic strategies in patients with acute decompensated heart failure. N Engl J Med. 2011;364:797–805.
4Mullens W, Damman K, Harjola VP, et al. The use of diuretics in heart failure with congestion — a position statement from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2019;21:137–155.
5Mullens W, Verbrugge FH, Nijst P, et al. Rationale and design of the ADVOR (Acetazolamide in Decompensated Heart Failure with Volume Overload) trial. Eur J Heart Fail. 2018;20:1591–1600.


 April 28, 2023
April 28, 2023