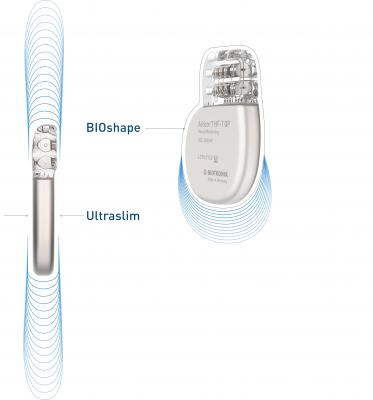
April 18, 2019 – Biotronik announced the European market release of what it calls the world’s smallest implantable cardioverter defibrillator (ICD) and cardiac resynchronization therapy defibrillator (CRT-D) devices that are approved for 3T full-body magnetic resonance imaging (MRI) scans.1
Devices in the Acticor and Rivacor families are only 10 mm slim, with a smooth and elliptical BIOshape that facilitates the insertion procedure. “The slenderness and smoother shape of the new devices play a key role in easing the insertion procedure and improving how the device looks after implantation,” reported Iain Matthews, M.D., Northumbria Healthcare, U.K., following his first implantations of the devices. More than 90 percent of implanting physicians rated “patient comfort” as “better” or even “much better” in comparison with previous models during post-market observation.2
Besides their body-friendly shape, Acticor and Rivacor devices feature an extended battery life, with up to 15 years for ICDs3 and nine years for CRT-Ds4. Increased device longevity is designed to lower the need for device replacements, resulting in reduced risks5, less distress for patients and fewer procedure costs. The new range of devices are also supported by a fully warranty of 10 years for ICDs and six years for CRT-Ds.
With Biotronik Home Monitoring, cardiovascular data from an Acticor or Rivacor device can be transmitted to the physician on a daily basis with programmable alerts about relevant changes in patient health and device status. The IN-TIME randomized controlled trial has demonstrated more than 60 percent reduction in all-cause mortality when CRT-Ds are used with Biotronik Home Monitoring.6 In ICDs, Home Monitoring has been clinically proven to help physicians detect atrial fibrillation earlier7, as well as reduce the number of inappropriate shocks by 90 percent and related hospitalization rates by 73 percent8. Biotronik Home Monitoring’s new QuickCheck feature gives access to patient data typically within three to four minutes.9
The typical non-response rate of 30-40 percent of all patients is a major problem in CRT.10,11 To counter this, Acticor and Rivacor CRT-Ds feature CRT AutoAdapt to fit patients’ individual pacing needs and provide continuous CRT adaptation – automatically adjusting to changes sensed in a patient’s condition every minute. Auto LV VectorOpt further helps to evaluate clinically relevant pacing parameters, with automatic threshold measurements across 20 vectors12 in less than 2.5 minutes13 and rapid direct programming.
All Acticor and Rivacor devices feature ProMRI, giving patients full access to high-resolution 3T MRI without any exclusion zone. With sensor-based MRI AutoDetect technology, once activated for a programmable window of up to 14 days, the devices can automatically recognize an MR environment and switch in and out of MRI mode as required. This ensures that patients receive optimal therapy, with tachycardia therapy suspension limited to the duration of the MRI scan, as well as fewer in-office visits required for device programming. Acticor and Rivacor devices also feature DX technology, enabling complete atrial diagnostics without an atrial lead.
For more information: www.biotronik.com
References
1. As part of an MR conditional system.
2. Post-Market observation; final-report, March 1, 2019. Data on file.
3. Acticor/Rivacor VR-T Standard conditions. 15.4 years @ 40 ppm; 0% pacing @ 2.5V/0.4ms; 500 Ohms; 2 max. energy shock/year. Data on file.
4. Acticor/Rivacor HF-T QP, 9.3 years @ 60 ppm; RA 15%, RV/LV 100% pacing, RA/RV/LV @ 2.5 V/0.4 ms; 500 Ohms, 2 max. energy shocks/year. Data on file.
5. Polyzos KA et al. Europace. 2015, 17(5).
6. Hindricks G et al. The Lancet. 2014, 384(9943).
7. Varma N et al. Circulation. 2010, 122(4).
8. Guedon-Moreau L et al. J Cardiovasc Electrophysiol. 2014, 25(7).
9. Performance analysis. Data on file.
10. Daubert C et al. Eur Heart J. 2017, 38(19).
11. Auricchio A et al. J Am Coll Cardiol. 2008, 51(15).
12. QP models only.
13. Post-Market observation; final report, March 1, 2019. Data on file.


 July 21, 2025
July 21, 2025 









