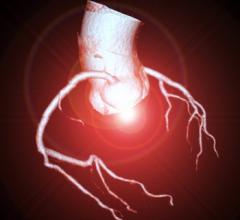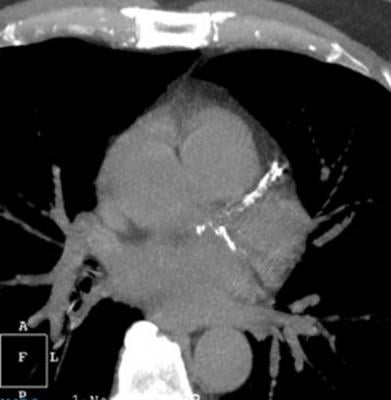
December 28, 2017 — HeartFlow Inc. announced that Health Care Service Corp. (HCSC), which operates Blue Cross and Blue Shield plans in five states, has issued a medical policy for the HeartFlow FFRct Analysis, a non-invasive technology that helps clinicians diagnose and treat patients with suspected coronary artery disease (CAD). HCSC — which operates Blue Cross Blue Shield plans in Illinois, Montana, New Mexico, Oklahoma and Texas — has determined that the use of noninvasive fractional flow reserve (FFR) following a positive coronary computed tomography (CT) angiogram may be considered medically necessary to guide decisions about the use of invasive coronary angiography in patients with stable chest pain at intermediate risk of CAD.
Additionally, BlueCross BlueShield companies in Tennessee, Louisiana and South Carolina recently issued a positive medical policy for the HeartFlow FFRct Analysis. After reviewing the technical performance, diagnostic accuracy, clinical utility and clinical benefits, all three entities concluded that the use of the HeartFlow FFRct Analysis following a coronary CT angiogram may be considered medically necessary for its members with stable chest pain and results in meaningful improvements in the net health outcome.
HCSC plans collectively cover nearly 15 million members across five states. BCBS of Tennessee covers 3.4 million members, Blue Cross and Blue Shield of Louisiana covers 1.6 million members, and BCBS of South Carolina covers approximately 850,000 people in the state.
The Centers for Medicare & Medicaid Services (CMS) and a growing number of commercial payers and professional organizations have recognized the value of the HeartFlow FFRct Analysis in diagnosing patients with suspected CAD, according to the company. CMS finalized a New Technology Ambulatory Payment Classification (APC) for the HeartFlow FFRct Analysis in November. Beginning Jan. 1, 2018, hospitals that are enrolled in Medicare and bill CMS will be able to submit claims for the HeartFlow FFRct Analysis for Medicare patients.
In addition to the new private payers, Anthem, Aetna and several other Blue Cross Blue Shield companies recently have issued positive medical coverage decisions. Evidence Street, which conducts healthcare technology evaluations for the Blue Cross Blue Shield Association, a national federation of 36 independent Blue Cross and Blue Shield companies, issued a positive review. In the U.K., the National Institute for Health and Care Excellence (NICE) of the National Health Service, which covers 64.6 million lives, also issued positive guidance. The American College of Cardiology (ACC) and American Heart Association (AHA) released updated Appropriate Use Criteria for Coronary Revascularization in Patients with Stable Ischemic Heart Disease. These criteria include the use of HeartFlow FFRct Analysis in determining the appropriateness of revascularization in many clinical scenarios.
For more information: www.heartflow.com
Related Content
CMS Will Pay For FFR-CT Noninvasive Coronary Analysis
Analysis Shows Better Outcomes, Cost Effectiveness Using FFR in Stable Coronary Disease

 February 06, 2026
February 06, 2026