October 27, 2017 — CorInnova Inc. announced it has received notice of allowance of a seminal patent to protect its intellectual property associated with the world’s first minimally invasively-delivered soft robotic heart device to support heart function. The company also announced that it has become a resident at Johnson & Johnson Innovation, JLABS at the Texas Medical Center (JLABS @ TMC). This residency follows a $6.1 million funding from the Wellcome Trust, details of which have not been disclosed.
CorInnova’s EpicHeart heart failure treatment technology includes the following features:
- First collapsible and self-expanding thin film soft robotic device for cardiac assist;
- Rapid minimally invasive implantation ability and simple delivery tool;
- Intrinsic pneumatic attachment inside the pericardial sac (no sutures or incisions to the heart or aorta);
- Non-blood-contacting device operation;
- Likelihood of up to 30-40 percent fewer adverse events than blood-contacting assist devices;
- Biventricular (or normal left ventricular) assist capability;
- Non-obligatory operation;
- Promotion of heart rehabilitation by promoting correct cardiac motion; and
- The potential to prevent the development of heart failure after major heart attacks.
CorInnova’s operations are located at JLABS @ TMC. JLABS is a 34,000 square-foot life science innovation center located in Houston. The labs provide a flexible environment for start-up companies pursuing new technologies and research platforms to advance medical care. Through a "no strings attached" model, JJI does not take an equity stake in the companies occupying JLABS, and the companies are free to develop products — either on their own, or by initiating a separate external partnership with JJI or any other company.
Further validation of the EpicHeart technology occurred in September, when CorInnova was the winner of a $50,000 award at the Fifth Annual Pediatric Device Innovation Symposium organized by the Sheikh Zayed Institute for Pediatric Surgical Innovation at Children’s National Health System and funded by the U.S. Food and Drug Administration (FDA) National Capital Consortium for Pediatric Device Innovation. This competition is designed to foster innovation that will advance pediatric healthcare and address unmet surgical and medical needs for children.
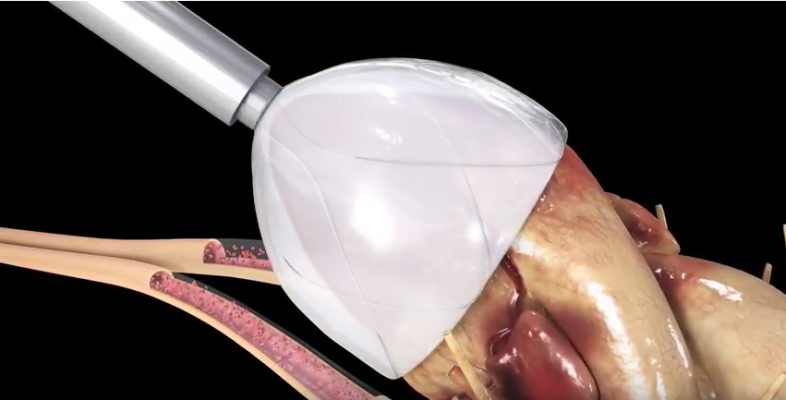
CorInnova has developed a direct cardiac compression device whose technology is a significant break with the prior art. CorInnova’s biventricular device is a collapsible thin-film pneumatically actuated soft robotic device that surrounds both ventricles of the heart. Air inflates the device in synchrony with the heart and increases cardiac output by gently squeezing the heart. CorInnova has also developed an innovative collapsible self-expanding device design that simplifies and speeds implantation. Due to the minimally invasive technology, hospital stays could potentially be reduced from 30 days to 4 to 6 days, compared to left ventricular assist devices (LVADs). CorInnova’s device can potentially be used for a range of end-stage heart failure patients for cardiac assist, ranging from short-term “bridge to decision” use, medium-term “bridge to transplant” use, and all the way to permanent “destination therapy” use. Diastolic as well as systolic heart failure patients may benefit from the technology. Diastolic heart failure patients currently have no approved device treatment.
The company was selected to present the EpicHeart technology as part of the "Shark Tank" competition at the 2017 Transcatheter Cardiovascular Therapeutics (TCT) annual scientific symposium, Oct. 29-Nov. 2 in Denver.
For more information: www.corinnova.com


 May 30, 2025
May 30, 2025