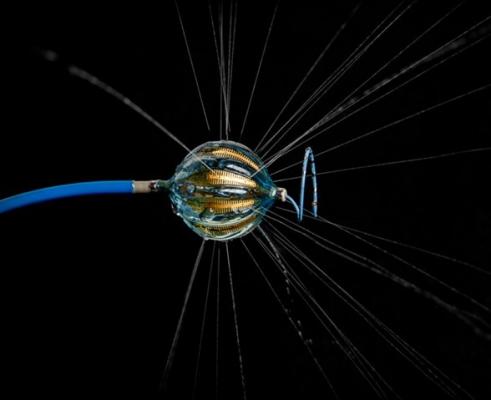
November 30, 2018 — Johnson & Johnson Medical Devices Companies announced that Biosense Webster Inc. has enrolled and treated the first patient in its STELLAR U.S. Investigational Device Exemption (IDE) study. The study will evaluate the safety and effectiveness of Heliostar Multi-electrode Radiofrequency (RF) Balloon Ablation Catheter in treating symptomatic drug refractory recurrent paroxysmal (intermittent) atrial fibrillation (AF). Up to 640 patients will be enrolled in as many as 40 clinical sites worldwide.
"This new balloon catheter is unique because it conforms to any pulmonary vein anatomy and allows me to control electrodes individually to deliver tailored energy when ablating around pulmonary veins," said cardiac electrophysiologist Rodney Horton, M.D., who treated the first patient in the study with Andrea Natale, M.D., FHRS, FACC, FESC, at the Texas Cardiac Arrhythmia Institute at St. David's Medical Center.
"The Heliostar catheter design has the potential to overcome the limitations of current balloon ablation catheters, result in fewer catheter exchanges and, most importantly, shorter procedure times. Heliostar is an exciting technology and we look forward to seeing the final study results," said Natale, cardiac electrophysiologist and executive medical director, Texas Cardiac Arrhythmia Institute at St. David's Medical Center+.
The Heliostar RF Balloon Ablation Catheter has 10 electrodes, which allows electrophysiologists to deliver different levels of energy depending on the tissue during lesion creation. In addition, the balloon design makes it possible to achieve pulmonary vein isolation with a single application of RF energy. The device is compatible with the Biosense Webster CARTO 3 Mapping System, an advanced imaging technology that enables creation of real-time 3-D maps of a patient's cardiac structures. The use of the CARTO 3 System during an ablation procedure can reduce exposure to radiation from fluoroscopy.
It is estimated that 33 million people worldwide are living with AF, or an irregular heartbeat, which can lead to blood clots, stroke, heart failure and other heart-related complications.1
For more information: www.biosensewebster.com
Reference


 February 06, 2026
February 06, 2026 









