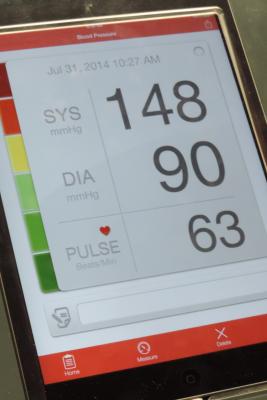
October 29, 2018 — Blood pressure readings of 130/80 millimeters of mercury (mmHg) or higher taken at home can be used to diagnose hypertension in white, black and Hispanic U.S. adults, according to new research in the American Heart Association’s journal Hypertension.1
“Until now, recommendations for diagnosing high blood pressure with measurements done at home were primarily from Japanese and European studies,” said Wanpen Vongpatanasin, M.D., study author, professor of medicine and hypertension director at UT Southwestern Medical Center in Dallas. “We didn’t know if these recommendations actually applied to U.S. adults.”
Researchers analyzed large multi-ethnic studies that compared home blood pressure to clinic measurements of primarily young and middle-aged adults in Dallas, Texas and Durham, N.C. In 420 participants in the North Carolina clinic, high blood pressure readings (130/80) were confirmed with similar readings at home. In 3,132 participants in the Dallas study, researchers determined risks of stroke, heart attack and death associated with a clinic systolic blood pressure reading of 130 mm Hg. During the 11-year follow up, researchers also determined that people with high blood pressure levels measured at home had the same heart disease risk as people with similar levels measured by medical professionals.
Vongpatanasin added that the findings correlate with the American Heart Association/American College of Cardiology 2017 blood pressure guideline.
“It’s important to measure blood pressure at home because clinic readings might not reflect a person’s true blood pressure. Some people have higher readings in the clinic because of the ‘white coat’ phenomenon, while studies have shown that others — especially blacks — have lower blood pressure readings in the clinic than at home,” she said.
This study included adults aged 30 to 65 years old from two U.S. cities, so the findings might not apply to younger or older people or adults in different geographical areas, she said.
With the definition of high blood pressure set at 130/80 mm Hg, nearly half (46 percent) of U.S. adults have high blood pressure. Researchers have found that at least 30 percent to 45 percent of U.S. adults with hypertension monitor their blood pressure at home.
The American Heart Association recommends consumers follow the proper technique when measuring blood pressure:
- Be still and rest quietly for at least 5 minutes before measurements;
- Avoid caffeine or cigarettes in the half hour before your reading;
- Keep both feet flat on the floor and void talking during the test;
- At least two readings should be taken one minute apart in the morning before taking medications and in the evening before eating; and
- Ideally, blood pressure readings should be taken during one week beginning a few weeks after a change in the treatment regimen and during the week before a doctor’s visit.
For more information: www.ahajournals.org/journal/hyp
Reference


 January 05, 2026
January 05, 2026 









