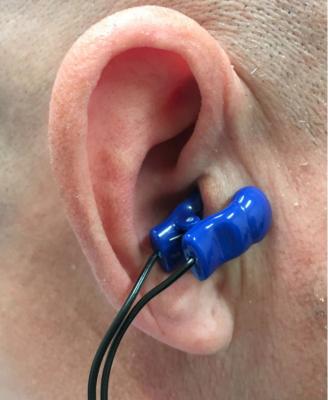
The Parasym Salustim device ear clip stimulates the vagus nerve, which was found to reduced AF burden compared with a sham procedure in the TREAT AF trial.
May 13, 2019 — A randomized clinical trial effectively used nerve stimulation through an ear clip to reduce atrial fibrillation (AF) burden and showcases an emerging noninvasive treatment option for patients. The trial is the first to examine the effect of transcutaneous electrical vagus nerve stimulation on suppressing AF. The results of the late-breaking TREAT AF trial were presented at Heart Rhythm 2019, the Heart Rhythm Society's 40th Annual Scientific Sessions.
Device vagus nerve stimulation has been successfully implemented to treat epilepsy and is a treatment option approved by the U.S. Food and Drug Administration (FDA). About one third of people do not respond fully to anti-seizure drugs and nerve stimulation has become an option for these patients.[1] The Parasym Salustim device used in this trial uses transcutaneous vagus nerve stimulation (TVNS) through a small clip. The device is used for treating tinnitus, but this trial sought to better understand if this treatment option could work for patients suffering from AF.
"Our clinical trial introduces a novel self-administered, non-invasive therapy for patients newly diagnosed with atrial fibrillation. There are cases when drug therapy or surgery are not the optimal options, so this has the potential to serve as another approach for patients," said lead author Stavros Stavrakis, M.D., Ph.D., University of Oklahoma Health Sciences Center. "It was encouraging to see the significant reduction in AF burden and that the device was well received in terms of adherence rate. This approach opens the door to a new way of thinking about AF management and showcases that we can push boundaries to help advance patient care."
The TREAT AF trial is a sham-controlled, double-blind, randomized clinical trial designed to examine the effect of low-level transcutaneous electrical stimulation of the auricular branch of the vagus nerve at the tragus (LLTS) on AF burden over a six-month period. LLTS was delivered using an ear clip attached to either tragus (active; n=26) or the ear lobe (sham; n=27). The patients received individual training and used the device for one hour every day for six months. AF burden over two-week periods was assessed by noninvasive continuous ECG monitoring at baseline, three and six months and heart rate variability based on five minutes ECG readings and serum cytokines were measured at the respective time points. Baseline characteristics including age, gender and stroke risk were similar between the two groups.
Adherence to the stimulation protocol was 75 percent in the active arm and 83 percent in the sham arm. At six months, the median AF burden was reduced by 85 percent in the active arm compared to the sham. After combining across the three- and six-month time points, the median AF burden was reduced by 75 percent in the active arm compared to the sham arm.
The authors of this study call for trials in a multi-center setting with larger populations. Through additional research, the authors also hope to pinpoint exactly which patients would benefit the most from this therapy approach.
AF is the most common abnormal heart rhythm condition. It affects more than 33.5 million people around the world and this number is expected to continue to rise.[2]
All the HRS 2019 late-breaking studies
References:
1. Epilepsy Foundation. Vagus Nerve Stimulation. Epilepsy.com. https://www.epilepsy.com/learn/treating- seizures-and-epilepsy/devices/vagus-nerve-stimulation-vns
2. Sumeet S. Chugh et al. Worldwide Epidemiology of Atrial Fibrillation. Circulation. 2014;129:837–847


 May 30, 2025
May 30, 2025