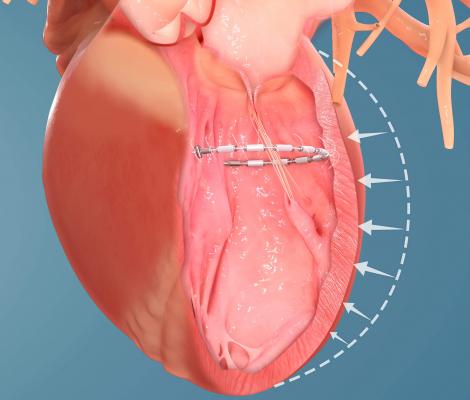
September 27, 2019 — Ancora Heart Inc. announced results from an interim analysis of heart failure patients treated in the CorCinch FMR study, a U.S. early feasibility study. CorCinch FMR evaluated the safety of the investigational AccuCinch Ventricular Repair System designed for the treatment of systolic heart failure. The data was presented at the 31st Transcatheter Cardiovascular Therapeutics (TCT), the annual scientific symposium of the Cardiovascular Research Foundation, Sept. 24-29 in San Francisco.
The latest available analysis of the first 31 heart failure patients with functional mitral regurgitation treated with the AccuCinch system at leading heart centers across the United States was presented by Satya Shreenivas, M.D., interventional cardiologist at The Christ Hospital Heart and Vascular Center and The Carl and Edyth Lindner Center for Research and Education at The Christ Hospital.
“Early safety and efficacy data on the transcatheter AccuCinch system is impressive and suggests the therapy has the potential to repair the left ventricle, improve heart function and restore the quality of life for heart failure patients,” said Shreenivas. “Notably, the results from patients at six-month follow-up indicates heart function improves over time. These initial results are highly promising and demonstrate AccuCinch’s potential to address many of the shortcomings of current heart failure therapies.”
Data from the interim analysis indicate a favorable safety profile with 97 percent freedom from device-related major adverse events at 30 days. Preliminary efficacy data from the first nine patients treated with the latest implantation technique and with adjudicated core lab data available through six months demonstrated a reduction in left ventricular volume by an average of 23 percent. Ejection fraction, a measure of blood flowing out of the left ventricle, improved on average from 31 percent to 39 percent over the same period. Additionally, Kansas City Cardiomyopathy Questionnaire scores increased by an average of 30 percent, suggesting reducing the left ventricular volume resulted in improved quality of life and reduced heart failure symptoms for this group. Further, mitral regurgitation grades and regurgitant volumes were both substantially reduced across this cohort.
Enrollment in the CorCinch FMR study recently concluded and 35 patients were treated with the AccuCinch at 15 heart centers. The primary safety endpoint of the study is device-related or procedure-related major adverse events through 30 days. Secondary exploratory endpoints include technical success, device and procedural success, as well as other observational endpoints measuring heart function, heart failure symptoms and changes in quality of life.
The transcatheter AccuCinch therapy is designed to complement and enhance the existing care cardiologists provide to further manage symptoms and slow, or stop, the progression of heart failure. For some patients, AccuCinch may have the potential to reverse the enlargement of the left ventricle. For patients where heart failure has progressed beyond the ability for medications and pacemakers to manage symptoms, non-surgical percutaneous device therapy with AccuCinch may provide an effective treatment option. The AccuCinch system is designed to directly repair the left ventricle of the heart, thereby addressing the fundamental issue in the progression of systolic heart failure.
For more information: www.ancoraheart.com


 January 05, 2026
January 05, 2026 









