Nov. 12, 2014 — Catalist-listed QT Vascular Ltd. has acquired a novel technology platform called Java, and all ...
Balloon Catheter
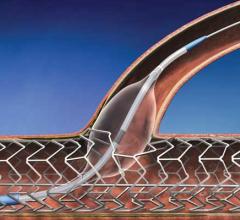
This channel includes news and new technology innovations for angioplasty balloon catheters (PTA). These are used in arteries with atherosclerotic lesions to compress the plaque expand the artery lumen to reopen occluded or heavily stenosed atherosclerotic lesions. Balloons are often used in combination with a stent to prop the treated vessel segment open. In addition to plain old balloon angioplasty (POBA), this section includes news about drug-coated balloon (DCB), valvuloplasty balloons and specialty cutting balloon.
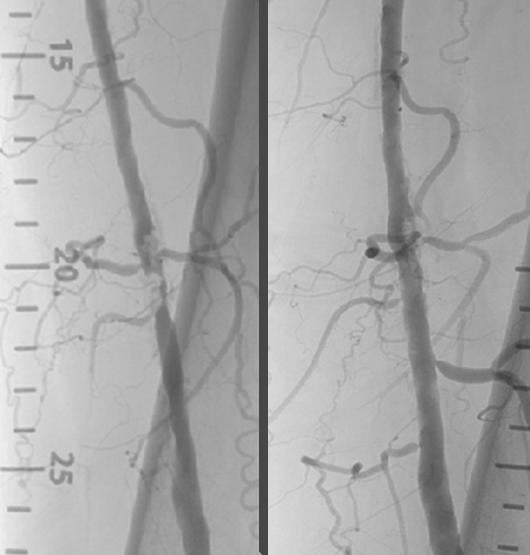
Nov. 11, 2014 — Shockwave Medical announced positive clinical results from Disrupt PAD trial, a single-arm multicenter ...
Nov. 10, 2014 — For the treatment of peripheral artery disease in leg arteries above the knee, the IN.PACT Admiral drug ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
November 4, 2014 — Shockwave Medical announced that the company will present clinical results from DISRUPT PAD, a single ...
October 31, 2014 — Metro Health (Michigan) cardiovascular specialist Jihad Mustapha, M.D., is one of the first ...
October 29, 2014 — Restenosis, the recurrence of narrowing of the arteries after stenting, is a common risk of this ...
October 14, 2014 — Presented for the first time at the 2014 Transcatheter Cardiovascular Therapeutics (TCT) conference ...
October 13, 2014 — C. R. Bard Inc. announced the U.S. Food and Drug Administration (FDA) approval of the Lutonix 035 ...

September 11, 2014 — Medtronic announced the U.S. Food and Drug Administration (FDA) 510(k) clearance and launch of the ...
August 12, 2014 — Teleflex Inc.’s subsidiary Hotspur Technologies Inc. received U.S. Food and Drug Administration (FDA) ...
July 28, 2014 — Boston Scientific Corp. received CE mark for the Ranger Paclitaxel-coated PTA Balloon Catheter. The ...
July 11, 2014 — Cordis Corp. announced the launch of its Saber PTA (percutaneous transluminal angioplasty) dilatation ...

June 16, 2014 — C. R. Bard Inc. announced that the U.S. Food and Drug Administration’s (FDA) Circulatory System Devices ...

 November 12, 2014
November 12, 2014