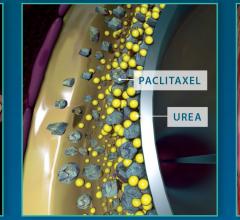
May 3, 2018 — The U.S. Food and Drug Administration (FDA) has expanded the indication for the Medtronic In.Pact Admiral ...
Balloon Catheter
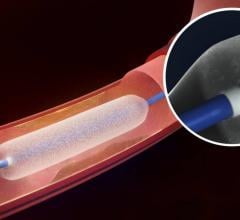
This channel includes news and new technology innovations for angioplasty balloon catheters (PTA). These are used in arteries with atherosclerotic lesions to compress the plaque expand the artery lumen to reopen occluded or heavily stenosed atherosclerotic lesions. Balloons are often used in combination with a stent to prop the treated vessel segment open. In addition to plain old balloon angioplasty (POBA), this section includes news about drug-coated balloon (DCB), valvuloplasty balloons and specialty cutting balloon.
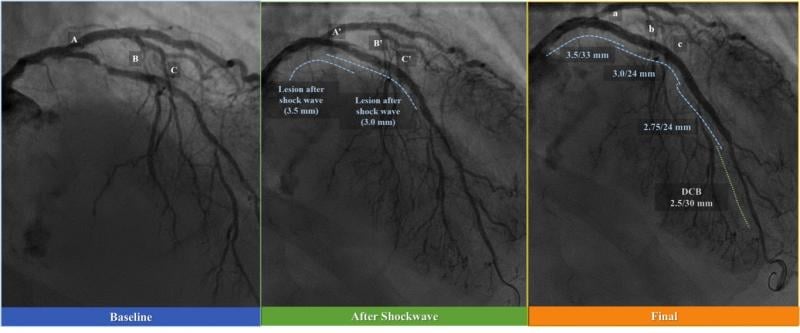
Over the last decade, there have been considerable developments in procedural techniques and technology facilitating the ...
March 21, 2018 — Cardiovascular Systems Inc. (CSI) recently announced that the U.S. Food and Drug Administration (FDA) ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
February 7, 2018 — Medtronic plc recently added to its body of clinical evidence supporting the In.Pact Admiral drug ...
January 24, 2018 — Cardiovascular Systems Inc. recently announced two new partnerships broadening the company’s product ...
December 29, 2017 — iVascular announced the release of the new Oceanus 14 Pro percutaneous transluminal angioplasty (PTA ...
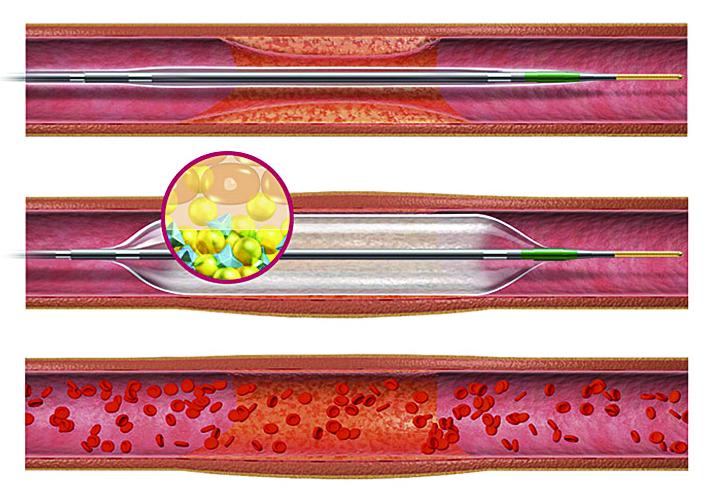
November 2, 2017 — The treatment of in-stent restenosis (ISR) remains challenging in clinical practice. The DARE (Drug ...
September 27, 2017 — Spectranetics is recalling its Bridge Occlusion Balloon Catheter due to the possibility of a ...
September 18, 2017 — Philips announced the two-year results from the ILLUMENATE European randomized clinical trial (EU ...
September 13, 2017 — Philips announced its presence at the Vascular Interventional Advances (VIVA 17) Annual Conference ...
September 13, 2017 — Medtronic plc announced that the In.Pact Admiral Drug-Coated Balloon (DCB) received approval from ...
August 29, 2017 — C.R. Bard Inc. announced the Lutonix 035 Drug Coated Balloon PTA Catheter (DCB) has been granted ...
August 15, 2017 — Surmodics Inc. announced receipt of an investigational device exemption (IDE) from the U.S. Food and ...
July 26, 2017 — The Spectranetics Corp. announced receipt of U.S. Food and Drug Administration (FDA) pre-market approval ...

June 28, 2017 — Philips Healthcare and Spectranetics Corp. announced they have entered into a definitive merger ...

 May 03, 2018
May 03, 2018