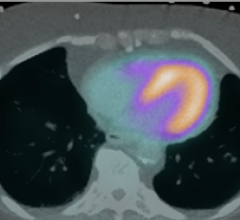
Nov. 17, 2025 — GE HealthCare has announced that Cardiovascular Associates of America (CVAUSA) plans to broaden its adoption of GE HealthCare’s FDA-approved cardiac positron emission tomography (PET) ...
PET-CT
PET-CT combines positron emission tomography (PET) detectors and computed tomography (CT) into one imaging system. PET requires the use of CT for image attenuation correction. The CT scanners that can be installed on these systems include 4-slice, up to workhorse 64-slice or higher systems. High slice CT is usually an option picked by hospitals that plan to use the PET-CT scanner for standard CT only imaging exams as well. The CT scan anatomical imaging can be fused with the nuclear PET imaging to show anatomical landmarks. The CT component can also be used in cardiac PET to perform a coronary calcium scoring exam to offer a risk assessment for future heart attack risk.
Nov. 17, 2025 — GE HealthCare has announced that Cardiovascular Associates of America (CVAUSA) plans to broaden its ...

Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
June 23, 2025 — GE HealthCare’s commitment to advancing precision care in cardiology through its molecular imaging ...
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
July 25, 2024 — Positron Corporation, a leading molecular imaging medical device company offering PET & PET-CT imaging ...
June 18, 2024 — Positron Corporation, a leading molecular imaging medical device company offering PET and PET-CT ...
June 14, 2024 — Positron Corporation, a leading molecular imaging medical device company offering PET and PET-CT(Positro ...
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
April 23, 2024 — CDL Nuclear Technologies, a pioneer in advanced diagnostic solutions, is proud to announce the launch ...

Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
October 5, 2023 — Jubilant DraxImage Inc., a wholly-owned subsidiary of Jubilant Pharma Limited, has entered into an ...
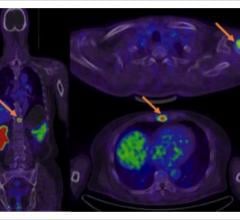
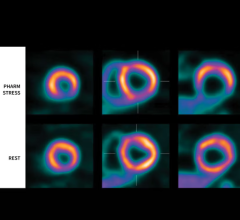
September 14, 2022 — GE Healthcare and Lantheus Holdings Inc have announced that the recent Phase III clinical trial of ...
September 7, 2022 — The American Society of Nuclear Cardiology (ASNC) and three partner societies have come together to ...
July 11, 2022 – The American Society of Nuclear Cardiology (ASNC) and the Society of Nuclear Medicine and Molecular ...
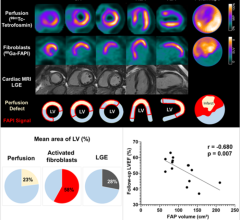
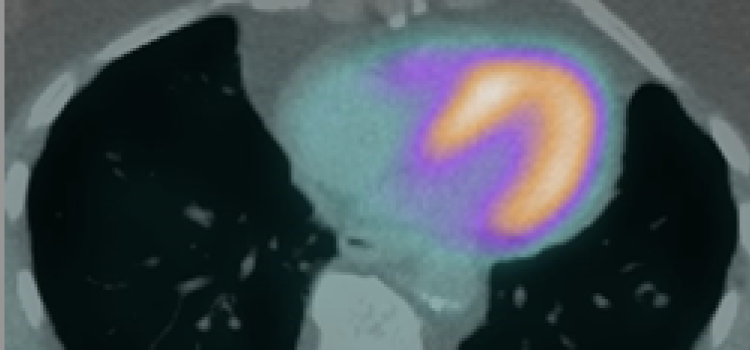
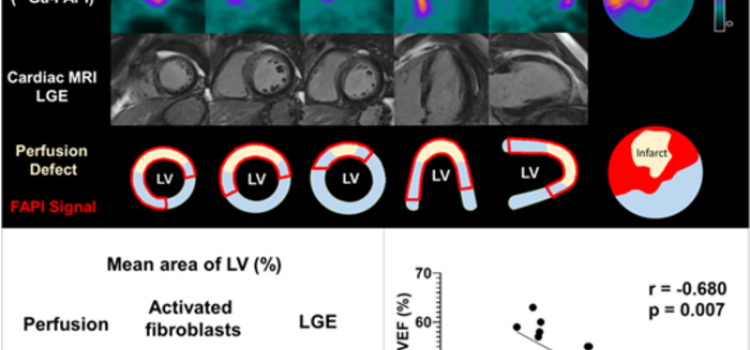
June 15, 2022 — Poor functional outcomes after a heart attack can be predicted with a new PET imaging agent, 68Ga-FAPI ...
July 13, 2021 — In a recent blog, the American Society of Nuclear Cardiology (ASNC) reported that Humana, one of the ...

A year after COVID-19 turned the world upside down, the American Society of Nuclear Cardiology (ASNC) asked members how ...


 November 17, 2025
November 17, 2025




![Phase III clinical trial of [18F]flurpiridaz PET diagnostic radiopharmaceutical meets co-primary endpoints for detecting Coronary Artery Disease (CAD)](/sites/default/files/styles/content_feed_medium/public/Screen%20Shot%202022-09-13%20at%203.30.13%20PM.png?itok=2w6OoNd6)