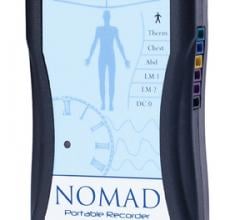
June 2, 2010 – A portable point-of-care prothrombin time/international normalized ratio (PT/INR…
June 1, 2010 – A home sleep testing technology eliminates the need for hospital sleep lab…
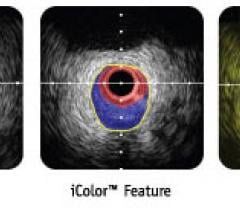
May 24, 2010 – New collaborations will enable the use of Boston Scientific’s iLab Ultrasound…
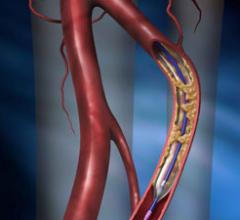
May 20, 2010 – A fractional flow reserve (FFR) guide wire gained U.S. Food and Drug…
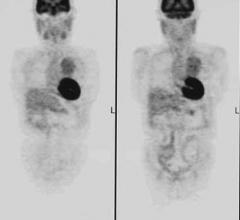
May 20, 2010 – A new partnership enables data communication between Medrad's Intego positron…
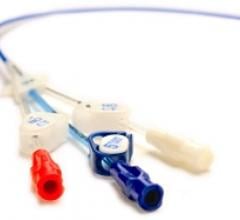
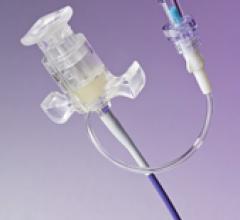
May 19, 2010 – Three distinct lumens in a new line of peripherally inserted central catheters (…
May 19, 2010 – A high-performance balloon dilatation catheter designed for use in peripheral…
May 18, 2010 – A pulse oximeter that integrates with a telehealth platform is designed for ease…
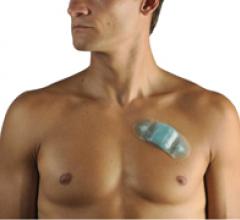
May 13, 2010 – An easy-to-wear, single-use, continuous ambulatory cardiac rhythm monitor yields…
May 13, 2010 – The U.S. Food and Drug Administration (FDA) has cleared a small pacemaker with…
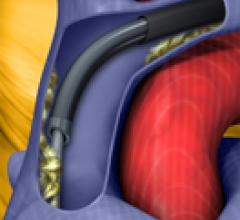
May 13, 2010 – An integrated, break-away hemostasis valve is integrated into a new lead delivery…
May 13, 2010 – A new simulation system to augment traditional procedural training for physicians…
May 11, 2010 – A cellular accessory has been added to an implantable cardiac device remote…
May 11, 2010 – A wireless USB adaptor allows patients with implantable cardiac devices to stay…
May 11, 2010 – An new iPad application integrates with and enhances the reporting and workflow…
May 11, 2010 – A software upgrade provides enhanced functionality, including the ability to view…
May 10, 2010 – A new metabolic stress testing system combines an integrated 12-lead…
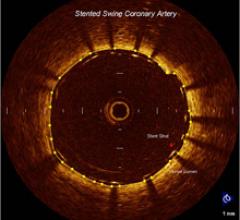
May 5, 2010 – A new high-definition intravascular imaging system gained U.S. Food and Drug…
May 3, 2010 – Two new ambulatory electrocardiogram (ECG) devices are being introduced this week…
April 28, 2010 – A Web-based platform designed to share electrocardiogram (ECG) information…


 June 02, 2010
June 02, 2010