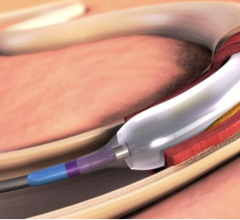
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
Balloon Catheter
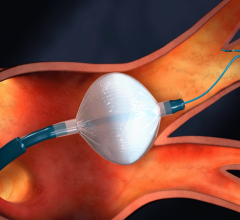
This channel includes news and new technology innovations for angioplasty balloon catheters (PTA). These are used in arteries with atherosclerotic lesions to compress the plaque expand the artery lumen to reopen occluded or heavily stenosed atherosclerotic lesions. Balloons are often used in combination with a stent to prop the treated vessel segment open. In addition to plain old balloon angioplasty (POBA), this section includes news about drug-coated balloon (DCB), valvuloplasty balloons and specialty cutting balloon.
April 28, 2023 — Medtronic today shared additional 36-month data from the IN.PACT AV Access Study, as well as 48-month ...
The European interventional cardiology device market is one of the larger markets in the global space. The market is ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
March 14, 2023 —MedAlliance has announced enrollment of over 1,000 patients in its ground-breaking SELUTION DeNovo coron ...
January 27, 2023 — The first US patient has been enrolled at Medstar Washington Hospital Center in the SELUTION4ISR ...
January 19, 2023 — Shockwave Medical, Inc., a pioneer in the development of Intravascular Lithotripsy (IVL) to treat ...
January 12, 2023 — Selution SLR, MedAlliance’s novel sirolimus-eluting balloon, has received conditional FDA ...
January 11, 2023 — In recent years, transcatheter mitral valve replacement (TMVR) treatment and technology has evolved ...
January 2, 2023 — The U.S. Food and Drug Administration (FDA) has approved the Stellarex 0.035” OTW Drug-coated ...
January 2, 2023 — The U.S. Food and Drug Administration (FDA) has approved the BioFreedom Drug Coated Coronary Stent (DC ...
|
November 10, 2022 — A national study led by UBC researchers at the Centre for Cardiovascular Innovation is shedding ...
November 3, 2022 — Medtronic, a global leader in medical technology, today announced the results of two clinical studies ...
October 28, 2022 — SELUTION SLR, MedAlliance’s novel sirolimus-eluting balloon, has received FDA Investigational Device ...
October 18, 2022 — Swiss-based medical technology company MedAlliance has announced it has entered into an agreement ...


 May 05, 2023
May 05, 2023