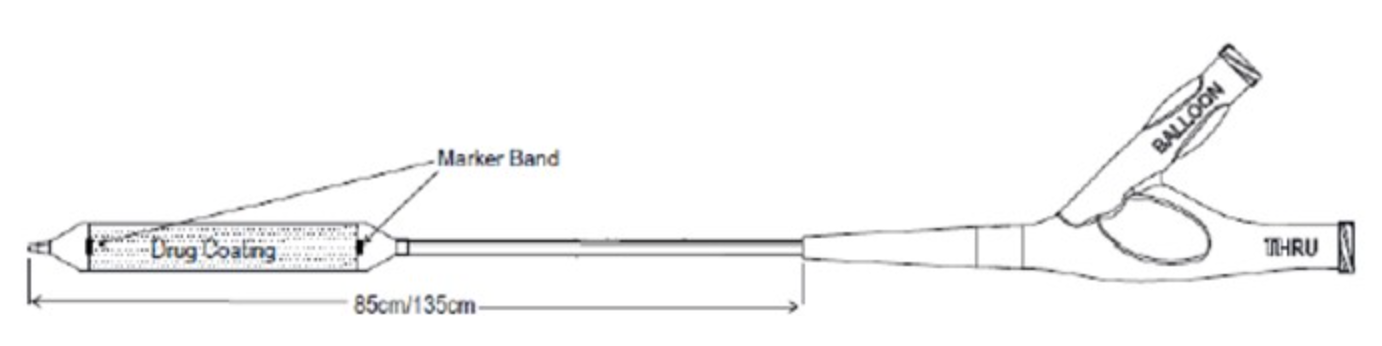
January 2, 2023 — The U.S. Food and Drug Administration (FDA) has approved the Stellarex 0.035” OTW Drug-coated Angioplasty Balloon – P160049/S015, from Philips Image Guided Therapy Corp. The Stellarex 0.035” OTW Drug-coated Angioplasty Balloon (Stellarex 035 DCB) uses a drug-coated balloon to re-open blocked or narrowed arteries in the thigh and knee due to peripheral artery disease (PAD). The balloon is coated on its outer surface with the drug paclitaxel which may help prevent the arteries from narrowing again (restenosis).
This approval expands the indications for use to include re-opening the part of a blood vessel that is within a previously placed stent.
A traditional angioplasty balloon catheter is inserted through the blood vessels, across the blockage or narrowing, and inflated to partially open the blocked or narrowed part. The Stellarex 0.035” OTW Drug-coated Angioplasty Balloon is then used to fully open the narrowed portion of the artery and apply the drug to the artery wall.
The Stellarex 0.035” OTW Drug-coated Angioplasty Balloon is used when arteries in the thigh and knee are narrowed or blocked as a result of peripheral artery disease. PAD occurs when fatty material (plaque) builds up in these arteries. The buildup can cause hardening and/or narrowing of the arteries (atherosclerosis), which limits the flow of oxygen-rich blood to the body. People with PAD may experience lifestyle-limiting symptoms, such as leg pain or serious complications, including skin ulcers or gangrene.
The Stellarex 0.035” OTW Drug-coated Angioplasty Balloon opens up a narrow or blocked artery in the thigh or knee. It can also be used to open up narrowing of a blood vessel within an already-placed stent. At 12 months, the Stellarex 0.035” OTW Drug-coated Angioplasty Balloon was able to keep 76.3% of arteries open. When used within a stent, the Stellarex 035 DCB was able to keep 58.8% of arteries open.
The Stellarex 0.035” OTW Drug-coated Angioplasty Balloon should not be used in:
- Patients with a known hypersensitivity (allergy) to paclitaxel or drugs with similar characteristics as paclitaxel.
- Patients who cannot take recommended blood-thinning or anti-clotting medicines.
- Women who are breastfeeding, pregnant, or intend to become pregnant and men who intend to father children.
- Arteries that carry blood to the heart (coronary), kidneys (renal), brain (cerebrovascular), or branch off from the largest artery (supra-aortic).
- If a doctor decides that the patient’s artery blockage will not allow complete inflation of the angioplasty balloon.
For more information: www.fda.gov
Related Drug-eluting Balloon Content:
VIDEO: Overview of Drug-coated Balloons — Interview with Juan F. Granada, M.D.
Comparison Chart of Drug-eluting Balloons (requires login but is free to signup)
Positive Data for the Ranger Drug-coated Balloon and Eluvia Vascular Stent
LEVANT Trial Data Shows Safety of Drug-Coated Balloon Shown
Drug-coated Balloon Maintains Good Outcomes in 4-Year IN.PACT Global Study Data
No Difference Between Drug-coated Balloons and Plain Balloons After Laser Atherectomy
Philips Shares Three-Year Results for Stellarex .035 Drug-Coated Balloon
VIDEO: SCAI Prospective on Key Takeaways at TCT 2019 — Interview with Chandan Devireddy, M.D., including discussion of the LEVANT study results


 October 24, 2025
October 24, 2025