Step 2/3
Drug-Coated Balloons
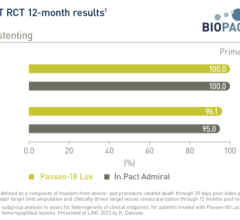
Drug-coated balloons (DCB), also referred to as drug-eluting balloons, are used to treat peripheral and coronary artery lesions and restenosis. The balloons carry an antiproliferative drug that is delivered to the wall of arteries when the balloon is expanded. The drug helps prevent neointimal hyperplasia caused by vessel trauma from the balloon angioplasty. Find news and video content on DCBs

 August 22, 2025
August 22, 2025