May 5, 2016 — Intact Vascular Inc. announced that positive twelve-month results from its Tack Optimized Balloon ...
Stents
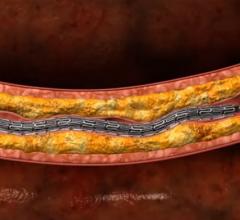
This channel includes news and new technology innovations for stents, also called vascular scaffolds. Stents are used to help prop open a vessel treated by balloon angioplasty because of the barotrauma caused by the extreme stretching of vessel walls. The stent enables to vessel to heal in an open position with collapsing. Drug eluting stents (DES) are coated in anti-proliferative drugs to precent scar tissue growth which can cause restenosis and occlude the vessel. DES require antiplatelet therapy because the drug carrier polymer on DES can cause thrombus inside the stent, even years after treatment, which is why bare metal stents are still used in some patients. This page includes news on coronary stents, carotid stents, peripheral stents, bioresorbable stents, and renal stents.
April 27, 2016 — Veniti Inc. announced the first successful treatment with the Vici Verto Venous Stent System of a ...
April 21, 2016 — Medtronic plc announced new clinical data from one of the endpoints in the RevElution Trial for its ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
April 18, 2016 — Delayed or deferred stent implantation in patients showed no clinical benefit in patients experiencing ...

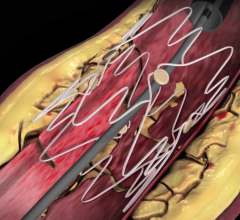
Bioresorbable stents have been one of the hottest new cardiovascular technologies discussed at cardiology meetings over ...
Gregg Stone, M.D., director of cardiovascular research and education at Columbia University Medical Center / New York ...
March 24, 2016 — Medinol announced the enrollment of their first patient in the U.S. NIRTRAKS Study. NIRTRAKS is a post ...
March 22, 2016 — PinnacleHealth CardioVascular Institute enrolled the first patient in Pennsylvania into the TOBA II ...
March 17, 2016 — Boston Scientific announced in late February that the Eluvia Drug-Eluting Vascular Stent System ...

March 15, 2016 — An independent panel of experts convened by the U.S. Food and Drug Administration (FDA) voted 9 to 0 ...
March 11, 2016 — Magnesium Elektron, developer, manufacturer and supplier of high-performance magnesium alloys ...
March 7, 2016 — Biotronik announced publication of results from the BIOSCIENCE trial in the journal EuroIntervention ...
March 4, 2016 — Recent results from the Carotid Revascularization Endarterectomy versus Stenting Trial (CREST) are ...
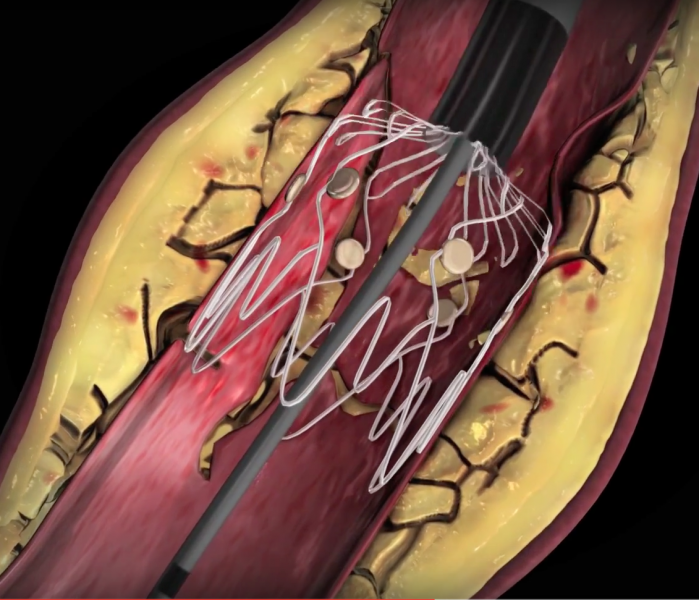
It is estimated that more than 10 million people in the United States are affected by peripheral arterial disease (PAD) ...
February 19, 2016 — Peripheral stents will account for over $4.6 billion in worldwide sales by 2020, according to a new ...


 May 05, 2016
May 05, 2016