Nov. 24, 2025 — InspireMD, Inc., developer of the CGuard Prime carotid stent system for the prevention of stroke, welcomes the recent presentation and publication of CREST-2 data which demonstrated ...
Stents
This channel includes news and new technology innovations for stents, also called vascular scaffolds. Stents are used to help prop open a vessel treated by balloon angioplasty because of the barotrauma caused by the extreme stretching of vessel walls. The stent enables to vessel to heal in an open position with collapsing. Drug eluting stents (DES) are coated in anti-proliferative drugs to precent scar tissue growth which can cause restenosis and occlude the vessel. DES require antiplatelet therapy because the drug carrier polymer on DES can cause thrombus inside the stent, even years after treatment, which is why bare metal stents are still used in some patients. This page includes news on coronary stents, carotid stents, peripheral stents, bioresorbable stents, and renal stents.
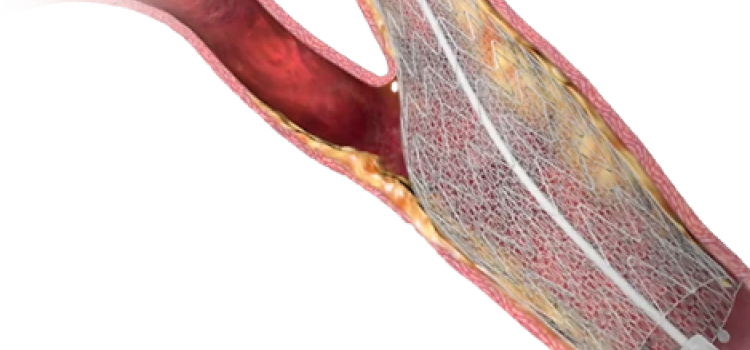
Jan. 6, 2026 — W. L. Gore & Associates’ medical business (Gore) has announced the FDA approval of the Gore Viabahn ...
Nov. 24, 2025 — InspireMD, Inc., developer of the CGuard Prime carotid stent system for the prevention of stroke ...
Oct. 13, 2025 — Medtronic plc recently announced it has received U.S. Food and Drug Administration (FDA) labeling ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
July 9, 2025 — InspireMD, Inc., developer of the CGuard Prime carotid stent system for the prevention of stroke ...
Oct. 16, 2024 — Sahajanand Medical Technologies (SMT) recently announced the publication of the COMPARE 60/80 HBR trial ...
July 11, 2024 — Medical device company R3 Vascular Inc., developer of novel, best-in-class bioresorbable scaffolds for ...
July 2, 2024 — Biotronik announced the availability of an expanded Maximum Allowed Diameters (MAD) range for the Orsiro ...
June 12, 2024 — Royal Philips, a global leader in health technology, announced the first implant of the Duo Venous Stent ...
June 4, 2024 — A patient at HonorHealth Research Institute is one of the nation’s first — and the first in Arizona and ...
June 3, 2024 — Elixir Medical, a developer of transformative technologies to treat cardiovascular and peripheral disease ...
May 30, 2024 — Biotronik announced the presentation of the 12-month results from the BIONETIC-I study this week at LINC ...
May 7, 2024 — HonorHealth Research Institute’s David G. Rizik, M.D., narrates and is a co-producer of a documentary ...
May 6, 2024 — SMT (Sahajanand Medical Technologies), a leading medical device company at the forefront of innovative ...
April 17, 2024 — Getinge and Cook Medical announced an exclusive sales and distribution agreement for the iCast covered ...
April 16, 2024 — Each year more than 500,000 Americans undergo percutaneous coronary intervention, or PCI, a minimally ...



 January 06, 2026
January 06, 2026