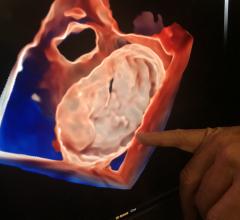
May 5, 2022 – Joe DiMaggio Children’s Hospital recently announced the successful treatment of a patient with atrial septal defects (ASD), or opening defect in the wall of the heart that separates the ...
Structural Heart Occluders
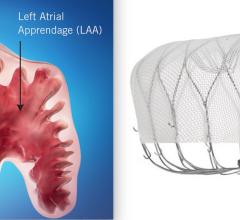
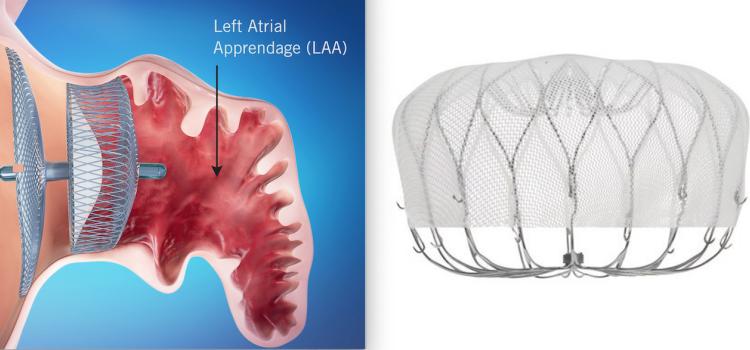
This channel includes news and new technology innovations about structural heart occluders. These include information of surgical repair and transcatheter closure of PFO, VSD, ASD and LAA. Devices include the Amplatzer and the Gore Helex
Dec. 18, 2025 — Abbott has received U.S. Food and Drug Administration (FDA) clearance and CE Mark for its Amplatzer ...
June 20, 2024 — atHeart Medical, a medical device company establishing a new standard of care for atrial septal defects ...
July 14, 2023 — In recent years, transcatheter intervention techniques have emerged as a promising alternative for the ...
February 27, 2023 — Conformal Medical, Inc. announced today the results from the CONFORMAL Early Feasibility Study (EFS) ...
May 5, 2022 – Joe DiMaggio Children’s Hospital recently announced the successful treatment of a patient with atrial ...
February 18, 2022 – Front Line Medical Technologies, Inc. today announced the expanded availability and distribution of ...
January 17, 2022 – As the increasing number of structural heart interventions are assisted by real-time imaging guidance ...
November 8, 2021 – SWISS-APERO is the first randomized clinical trial comparing the Abbott Amulet left atrial appendage ...
October 4, 2021 — U.S. Food and Drug Administration (FDA) has cleared the Abbott Amplatzer Talisman PFO Occlusion System ...
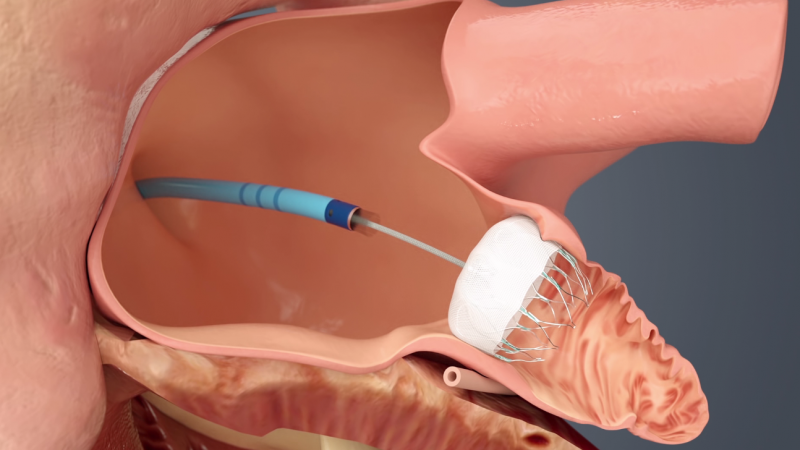
September 27, 2021 — Compared with men undergoing left atrial appendage occlusion (LAAO), women have a significantly ...
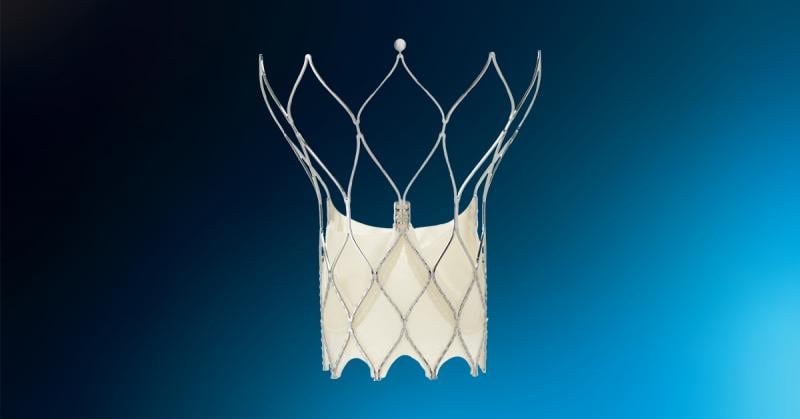
September 20, 2021 — Abbott announced that the U.S. Food and Drug Administration (FDA) has cleared the company's Portico ...
August 31, 2021 — Late-breaking data from a head-to-head clinical trial of the Amulet Left Atrial Appendage (LAA) ...
August 16, 2021 — The U.S. Food and Drug Administration (FDA) approved Abbott's Amplatzer Amulet Left Atrial Appendage ...
June 28, 2021 — CentraCare, one of the largest health systems in Minnesota, successfully completed the first structural ...
Tom Jones, M.D., director, cardiac catheterization laboratories, Seattle Children’s Hospital, explains some of the new ...





 December 18, 2025
December 18, 2025