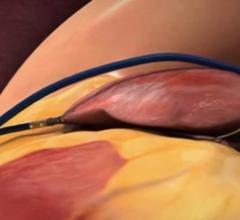
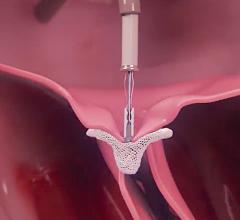
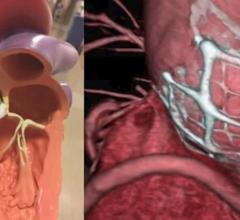
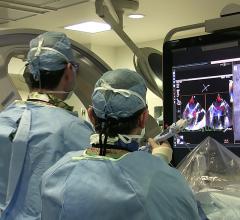
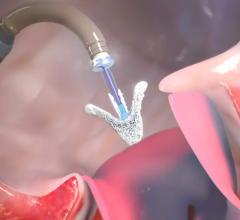
May 15, 2021 - Transcatheter left atrial appendage occlusion (LAAO) with a Boston Scientific Watchman device was ...
Structural Heart
This structural heart channel includes news, videos, podcasts and other content related to diagnosis and treatment of structural heart disease. Topics covered include heart valve repair and replacement, transcatheter aortic valve replacement (TAVR), transcatheter mitral valve replacement (TMVR), transcatheter tricuspid valve replacement (TTVR), left atrial appendage (LAA) occlusion, heart failure interventional device therapies, and closing holes in the heart using, including occlusion of atrial septal defects (ASDs), ventricular septal defects (VSDs) and patent foramen ovales (PFOs).

May 15, 2021 — Patients with an elevated risk of stroke due to heart rhythm problems, or atrial fibrillation (AFib) ...
May 15, 2021 — The anticoagulant apixaban (Eliquis) was not superior to standard of care following transcatheter aortic ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...

The mitral valve anatomy is extremely complex, which has caused many challenges for transcatheter mitral valve ...
Tom Jones, M.D., director, cardiac catheterization laboratories, Seattle Children’s Hospital, and principle investigator ...
Philippe Géneréux, M.D., director of the structural heart program at Atlantic Health System’s Morristown Medical Center ...
May 4, 2021 – A new study, presented at the 2021 American Association for Thoracic Surgery (AATS) 101st annual meeting ...
Ashwin Nathan M.D., a cardiology fellow at the Hospital of the University of Pennsylvania, presented a late-breaking ...
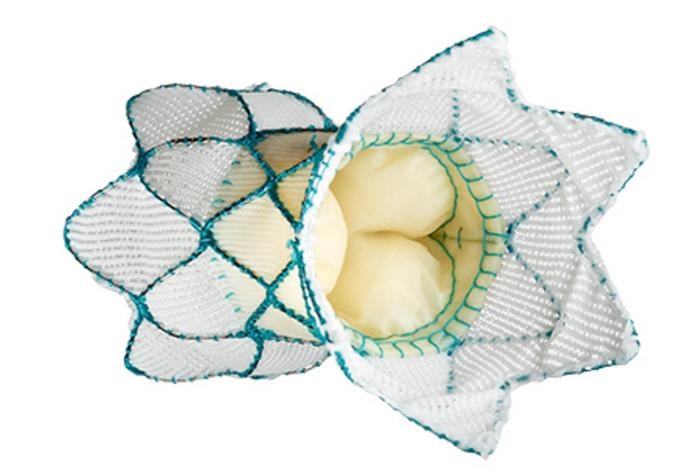
April 30, 2021 — New study results validate the effectiveness of the Medtronic Harmony transcatheter pulmonary valve ...
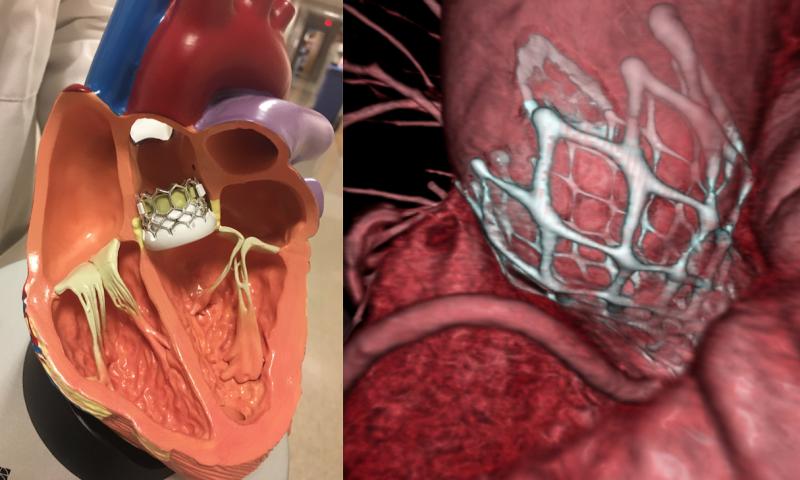
April 30, 2021 – An analysis of growth patterns in transcatheter aortic valve replacement (TAVR) programs across United ...
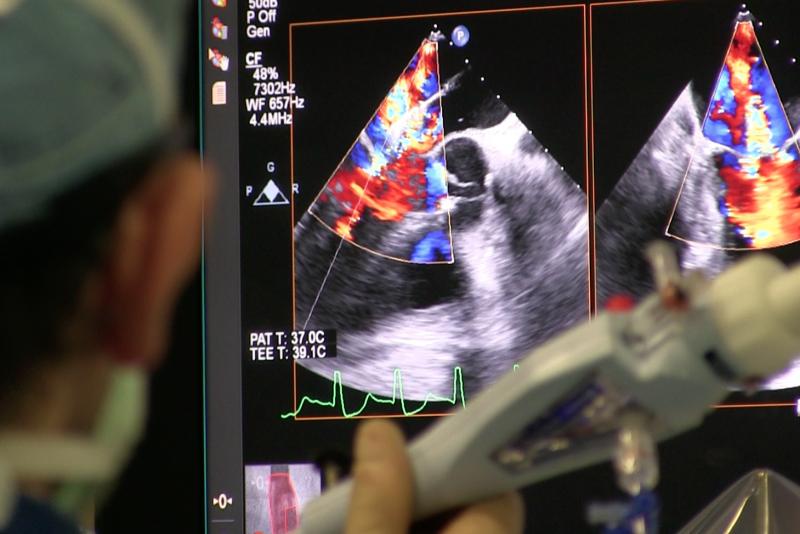
The resounding success of transcatheter aortic valve replacement (TAVR) has led the creation of hundreds of structural ...
As a medical technology journalist, a little more than a decade ago I found myself sitting in those late afternoon ...
April 20, 2021 — Start-up medical device company atHeart Medical announced it is initiating its U.S. investigational ...
April 14, 2021 — The U.S. Food and Drug Administration (FDA) cleared the Acutus Medical AcQCross family of universal ...
April 8, 2021 — Abbott today announced it has received European CE mark and Health Canada authorization this week for ...

 May 16, 2021
May 16, 2021