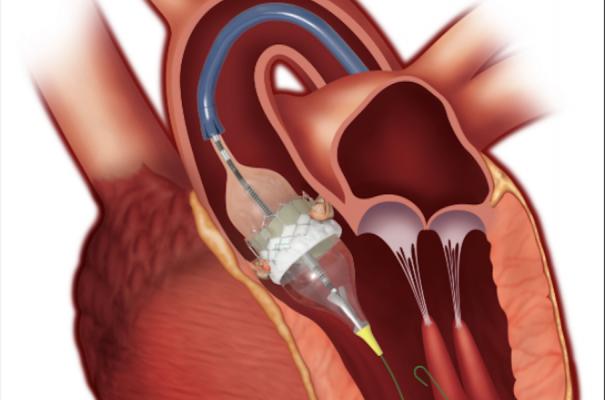
The Edwards Lifesciences Sapien 3 TAVR valve. The annual volume of TAVR has increased each year and in 2019 TAVR volume (72,991) exceeded all forms of SAVR (57,626), coinciding with the U.S. Food and Drug Administration (FDA) approval of TAVR for low-risk patients.
November 17, 2020 — Since the approval of the first transcatheter aortic valve replacement (TAVR) device in 2011, more than 276,000 patients have undergone a TAVR procedure in the United States. According to a report from The Society of Thoracic Surgeons (STS)/American College of Cardiology (ACC) TVT Registry, volumes have increased every year. In 2019 TAVR exceeded all forms of surgical aortic valve replacement (SAVR) for the first time.
In addition, over time the 30-day mortality and stroke rates have decreased, while pacemaker need remains largely unchanged. The full report was published Monday in the Journal of the American College of Cardiology and The Annals of Thoracic Surgery simultaneously.[1]
“The STS/ACC TVT Registry allows us to see major trends occurring in the real-world TAVR patient population, including a rapid growth in both the number of hospital sites performing TAVR and case volume as we treat a broader spectrum of patients. We have also seen TAVR become the leading choice for aortic valve replacement compared to the open surgical approach,” said John D. Carroll, M.D., chair of the STS/ACC TVT Registry Steering Committee and the lead author on the report. “Furthermore, the data on outcomes after TAVR document a substantial improvement in quality of care in the last nine years.”
Since the first TAVR device approval, access has been extended from patients considered inoperable or at extreme risk (2011), at high-risk (2012), intermediate-risk (2016) and low-risk (2019) for SAVR. Researchers examined data in the STS/ACC TVT Registry for all TAVR procedures performed at sites active through 2019. An early TAVR experience was defined as patients treated from late 2011 through 2013 and was compared to current TAVR experience, which was defined as patients treated in 2019.
Data in 2019 are from hospitals located in 49 U.S. states, as well as two sites in the District of Columbia and two sites in Puerto Rico. In August 2020 there were 715 U.S. TAVR sites. As of the opening of a site in Wyoming in 2020 TAVR programs exist in all 50 states. The only U.S. TAVR sites not included in the STS/ACC TVT Registry are those in military hospitals and the Veterans Authority (VA) medical system: as of mid-2019 there were eight VA TAVR programs.
Watch the related VIDEO: Tracking Transcatheter Valve Outcomes in the STS-ACC TVT Registry — Interview with John Carroll, M.D.
TAVR Procedure Volumes In the Unites States
The annual volume of TAVR has increased each year and in 2019 TAVR volume (72,991) exceeded all forms of SAVR (57,626), coinciding with the U.S. Food and Drug Administration (FDA) approval of TAVR for low-risk patients. The number of TAVR procedures performed per site varies, but as the number of sites performing TAVR has increased, the total annual volume has increased. In 2019 sites performed 84 TAVR procedures on average with 161 sites performing less than 50 cases. An expert consensus document published by stakeholders — including the ACC and STS — ncluded a recommendation of a 50-case annual threshold minimum for sites performing TAVR.
TAVR Patient Demographic Statistics
Data showed the median age of patients undergoing TAVR has slightly gone down from early TAVR experiences. In 2019 mostly males (56%) underwent TAVR, a shift away from the early TAVR period when there was a nearly equal male/female distribution of patients undergoing the procedure. For all years, patients undergoing TAVR were predominantly white with the persistence of under-representation of blacks, based on overall U.S. demographics, at 4% despite the substantial growth of sites offering TAVR. This finding will stimulate further research into its possible causes ranging from barriers to health care access to differences in the prevalence of disease. From 2011 thru 2018 extreme and high-risk patients remain the largest cohort undergoing TAVR, but in 2019 intermediate-risk was the largest cohort. In 2019, the first year TAVR was FDA-approved for low-risk patients, this population made up 11.5% of all TAVR patients and had a median age of 75.
Smaller TAVR Sheaths Allowed Large Increase in Femoral Access Procedures
In the early TAVR period with very large vascular sheaths required for the first-generation TAVR delivery catheters, only 57.1% of TAVR procedures used femoral artery access. By the current TAVR period in 2019, femoral access accounted for 95.3% of vascular access sites for the procedure. In 2019 the axillary-subclavian was the most commonly used alternative access approach, a shift from the use of transapical and direct aortic approaches that were the common alternative access point in the early TAVR period.
TAVR Length of Stay
The median length of stay for patients has declined from seven days to two days for all patients over the course of the report. For patients assessed as low-risk, the median length of stay in 2019 was only one-day—an overnight hospital stay—with some patients discharged the same day. In the early TAVR period, most patients were discharged to another care facility. In 2019 90.3% of patients were discharged home, while 6.6% were discharged to a rehabilitation or extended care facility and 2.45% to a nursing home.
U.S. TAVR Mortality, Stroke and Pacemaker Rates
Researchers also found a steady and dramatic shift in mortality from the early TAVR period to 2019. In-hospital mortality fell from 5.4 to 1.3% and 30-day mortality decreased from 7.2 to 2.5%. In-hospital and 30-day stroke rates also fell from the early TAVR period to the current TAVR period. However, the 30-day pacemaker implantation rate has remained largely the same. The in-hospital rate has fallen in the context of the shorter length of stay at the hospital.
TAVR Quality of Life Assessments
The STS/ACC TVT Registry has been innovative in gathering data from patients on their assessment of their individual health status, using the Kansas City Cardiomyopathy Questionnaire (KCCQ) before treatment. The registry also gathers data on whether there are changes post-TAVR in component metrics on quality of life (QOL), functional state and other patient reported outcomes. In 2018 — the most recent year with one-year outcomes data for patients treated on or before Sept. 30 — 80.7% of all patients who were alive at one-year post-TAVR and had complete KCCQ data reported a good QOL. In addition, a subgroup analysis revealed that this benchmark was achieved in 77.7% of high-extreme risk, 83.6% of intermediate-risk and 85.8% of low-risk patients.
According to the researchers, this report also provides a preliminary real-world assessment of patients having TAVR who were classified as being low-risk after the FDA approved these patients for TAVR in August 2019.
Related TAVR Content:
VIDEO: Tracking Transcatheter Valve Outcomes in the STS-ACC TVT Registry — Interview with John Carroll, M.D.
U.S. TAVR Outcomes Need Improvement Based on TVT Registry Analysis
Boston Scientific Pulls Lotus Edge TAVR Valve Off The Market
FDA Approves TAVR for Low-risk Patients Creates A Paradigm Shift in Cardiology
TAVR Expected to See Rapid Growth in Next 5 years
Boston Scientific Launches Acurate neo2 Transcatheter Aortic Valve System in Europe
Acurate neo TAVR Valve Fails to Meet Noninferiority With Medtronic CoreValve Evolut
VIDEO: Interventional Structural Heart Advances Are Rapidly Expanding — Interview with Juan F. Granada, M.D.
Boston Scientific Launches Acurate neo2 Transcatheter Aortic Valve System in Europe
TAVR Non-Inferior to Surgery in U.K. Review of Surgery vs. TAVR
Reference:


 November 14, 2025
November 14, 2025 









