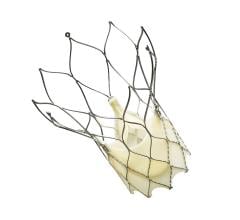
September 28, 2018 — Heart failure patients who received the Carillon Mitral Contour System in the REDUCE FMR clinical ...
TCT
This channel contains news about the annual Transcatheter Cardiovascular Therapeutics (TCT) conference presented by the Cardiovascular Research Foundation (CRF). It includes coverage from the annual meeting and links CRF news. TCT is the premier conference for the subspecialty of interventional cardiology, including the new subspecialty areas of transcatheter structural heart procedures.
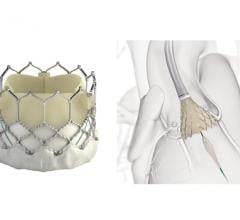
September 28, 2018 — At one year following transcatheter aortic valve replacement (TAVR), implantation with the Portico ...
September 28, 2018 — The first randomized study to compare general versus local anesthesia during transcatheter aortic ...
The optimal delivery of cardiac care is evolving rapidly. A growing number of patients combined with innovative new ...
Philippe Genereux, M.D., co-director of the structural heart program at the Gagnon Cardiovascular Institute at ...
September 27, 2018 — Biotronik recently announced U.S. Food and Drug Administration (FDA) approval of the PK Papyrus ...
Ron Waksman, M.D., associate director of the division of cardiology and director of cardiovascular research and advanced ...
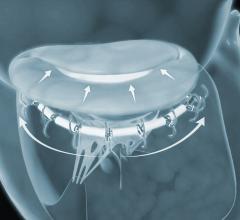
September 26, 2018 — Patients with heart failure and secondary mitral regurgitation (MR) who remained symptomatic ...
September 26, 2018 — Positive 12-month data from the late-breaking IMPERIAL trial was presented at the 2018 ...
September 25, 2018 – New data demonstrate the correlation between the presence of non-flow-limiting, non-intervened-upon ...
September 25, 2018 — Orchestra BioMed Inc. announced the three-year clinical results from its Sirolimus Angioplasty ...
September 24, 2018 — Ancora Heart Inc. announced positive clinical data from the company’s recently expanded U.S. early ...
September 24, 2018 — New data announced at the 2018 Transcatheter Cardiovascular Therapeutics (TCT) conference, Sept. 21 ...
September 24, 2018 — Corindus Vascular Robotics Inc. announced the first live transmission of a remote interventional ...
September 21, 2018 — Osprey Medical announced a collaboration with GE Healthcare on Osprey’s Be Kind to Kidneys campaign ...
September 21, 2018 — At the 2018 Transcatheter Cardiovascular Therapeutics (TCT) conference, Sept. 21-25 in San Diego ...


 September 28, 2018
September 28, 2018