Sept. 10, 2024 — Royal Philips and the World Stroke Organization (WSO) have published a policy paper calling for a ...
Thrombectomy Devices
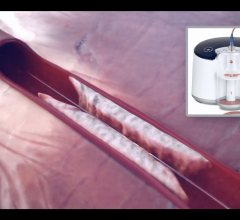
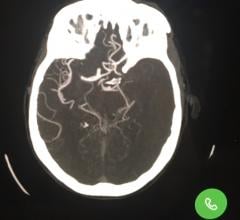
This channel includes news and new technology innovations for catheter-based thrombectomy systems used to remove blood clots from vessels in the body. Thrombus removal a decade ago was a standard of care for acute coronary revascularization in ST-elevation myocardial infarction (STEMI). But, its use declined rapidly after several large trials showed no benefit. However today, thrombectomy is seeing increasing usage in new therapeutic areas to treat acute stoke, pulmonary embolism (PE), deep vein thrombosis (DVT) and venous thromboembolism (VTE). Thrombectomy is also referred to as embolectomy.
The types of thrombectomy systems include:
● Ultrasound-assisted thrombolysis – Catheter-directed, high-frequency ultrasound helps thrombolytic agents penetrate clots to speed the action of fibrinolytic pharmacological therapy.
● Rheolytic embolectomy – These devices inject pressurized saline through the catheter's distal tip and the macerated thrombus is aspirated through a catheter port.
● Rotational embolectomy – A rotating device at the catheter tip is used to fragment the clot and fragments are aspirated by the catheter.
● Aspiration thrombectomy – This includes manual clot aspiration or use of dedicated aspiration catheter devices that basically vacuum the clot out of the vessel.
● Thrombus fragmentation – Thrombus can be mechanically disrupted by manually rotating a pigtail catheter or using balloon angioplasty, but this causes small fragments of emboli to flow distally. Dedicated devices also are available.
June 7, 2024 — Access to thrombectomy should be expanded to include patients who experience basilar artery occlusion ...
April 24, 2024 — Expanse ICE announced today the ICE Aspiration System has received 510(k) clearance from the U.S. Food ...
January 16, 2024 — Penumbra, Inc., a thrombectomy company, has recently secured CE Mark (Conformité Européenne) for its ...
January 23, 2023 — Imperative Care, Inc., announced U.S. Food and Drug Administration (FDA) 510(k) clearance of its Zoom ...
January 11, 2023 — The Alameda, Calif.-based global healthcare company Penumbra has announced U.S. Food & Drug ...
October 31, 2022 — Results from the Late-Breaking Clinical Trials session at The VEINS Conference in Las Vegas, NV, were ...
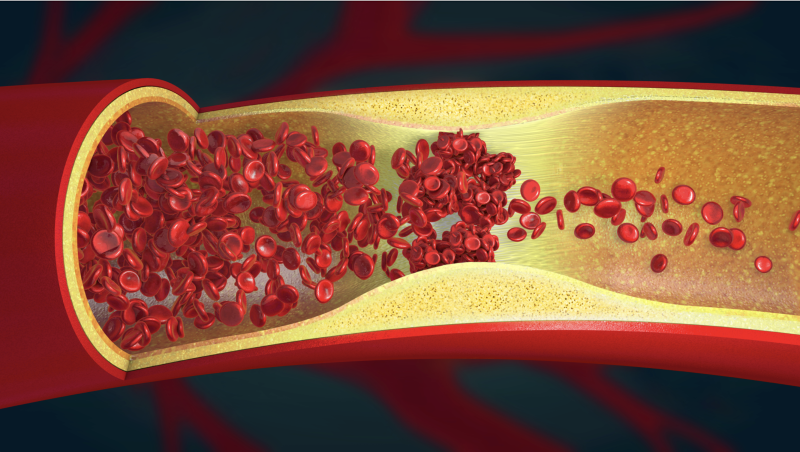
The global thrombectomy devices market is poised to experience substantial expansion, owing to the emergence of ...
May 10, 2022 — The MIVI Neuroscience Q Aspiration Catheter incorporates a novel pusher wire design on its proximal end ...
January 10, 2022 – Akura Medical Inc., a Shifamed portfolio company, announced the closing of its $25 million Series A1 ...

Artificial intelligence (AI) has found a unique niche to help automate the activation of acute care teams for pulmonary ...
November 12, 2021 — Penumbra Inc. announced that the CHEETAH clinical study of its Indigo System CAT RX Catheter ...
August 9, 2021 — Boston Scientific Corp. has commenced enrollment in the HI-PEITHO clinical trial, a collaborative ...
Ehtisham Mahmud, M.D., division chief of cardiovascular medicine, director of interventional cardiology and the cardiac ...
April 7, 2021 — Market research firm GlobalData's new report, Neurovascular Thrombectomy Devices (Neurology Devices), sh ...

 September 10, 2024
September 10, 2024