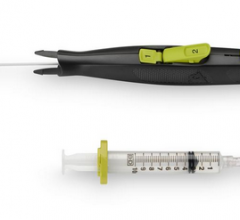
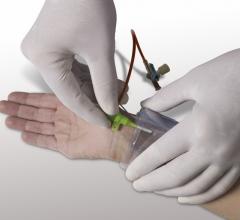
April 1, 2014 — AccessClosure Inc. commercially launched its Mynx Ace vascular closure device, a safe and secure ...
Vascular Closure Devices
This channel includes news and new technology innovations for vascular closure devices used to rapidly seal and achive hemostatsis at vascular access sites in interventional cardiology procedures.
March 21, 2014 — AccessClosure plans to launch the Mynx Ace Vascular Closure Device at the 2014 American College of ...
There is no shortage of debate among interventional cardiologists over whether vascular closure devices should be used ...
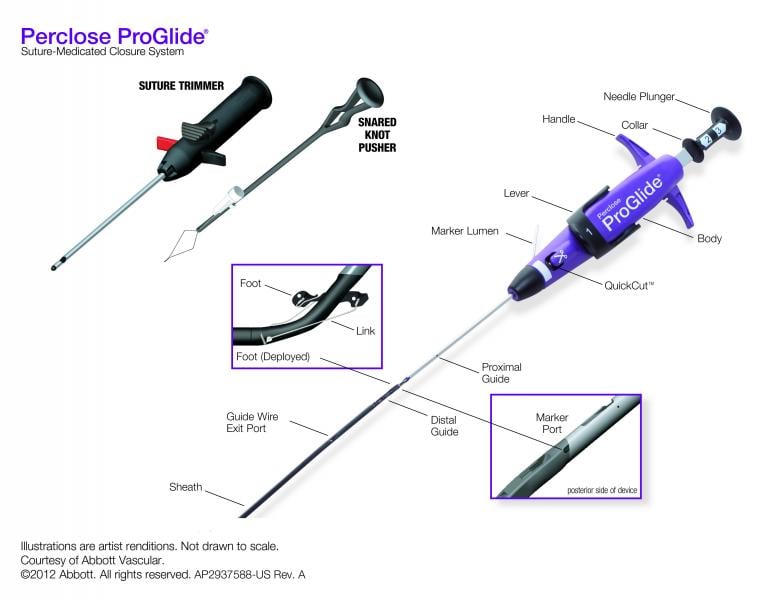
Vascular closure devices (VCD) were first introduced in the early 1990s with a goal to achieve quick hemostasis, thus ...
May 28, 2013 — The U.S. District Court for the District of New Jersey approved an agreement under which Vascular ...
February 19, 2013 — CardioLogical Solutions is an emerging cardiovascular device company formed by the merger of ...
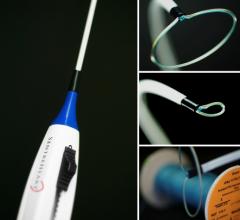
February 7, 2013 — Cardiva Medical Inc. announced that the U.S. Food and Drug Administration (FDA) has granted Premarket ...
December 10, 2012 — Vascular Closure Systems Inc. announced the successful conclusion of Phase I and Phase II of the ...
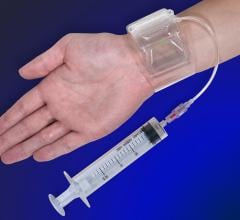
November 6, 2012 — Vascular Solutions launched the R-Band radial hemostasis compression device in the United States ...
Vascular closure devices that use an active method to immediately seal the femoral access site can enable faster patient ...
The implementation of dedicated access site surveillance and educational programs, in tandem with pre-existent ...
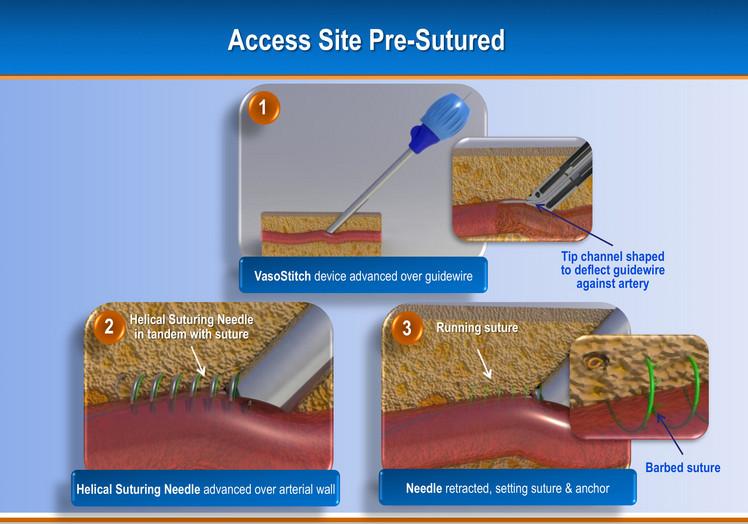
Due to the increasing number of transcatheter aortic valve replacements (TAVR) and endovascular aneurysm repair (EVAR) ...
July 6, 2012 — Vascular Closure Systems Inc. announced that the 30-day follow-up results for the Phase I first-in-human ...
June 4, 2012 — SentreHeart Inc., a privately held medical device company, announced that it has recently completed a $26 ...
May 4, 2012 - CardioDex announces initial commercial use of a new femoral access site closure device. Recent data from ...

 April 01, 2014
April 01, 2014