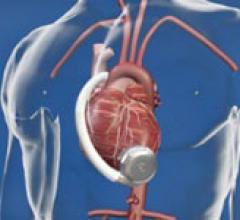
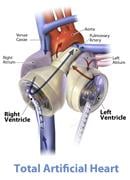
The practicality and expense of maintaining patients on total artificial hearts (TAH) may be significantly ...
Ventricular Assist Devices (VAD)
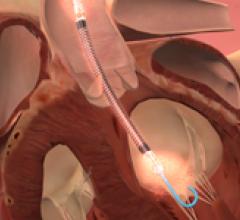
This channel includes news and new technology innovations for ventricular assist devices (VAD). VADs are a type of mechanical hemodynamic support device that helps increase blood flow in people who have ventricles that are not work properly due to heart failure, cardiogenic shock, cardiomyopathy or myocardial infarction. Most often these devices support the left ventricle, so they are often referred to as left ventricular assist devices (LVAD). VADS come in two types, surgically implanted, usually as a bridge to heart transplant, and percutanenous catheter-based pumps used for temporary hemodynamic support. Examples of temporary percutaneous pumps include the Impella and TandemHeart devices.
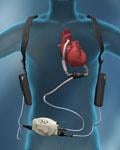
June 16, 2010 – A ventricular assist system has received conditional approval from the U.S. Food and Drug ...
New U.S. Food and Drug Administration (FDA) indications for two key heart failure device therapies may help ...
March 30, 2010 – The FDA granted an investigational device exemption (IDE) for a clinical study of the Freedom ...
March 9, 2010 – During the American College of Cardiology (ACC) 59th Annual Scientific Session, March 14-16 ...

The intra-aortic balloon pumps (IABPs) have been the gold standard for minimally invasive circulatory support for ...
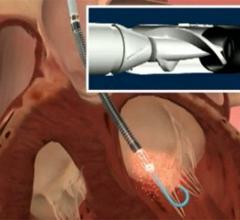
January 27, 2010 – Robust blood pressure measurements via fiberoptic sensors will be added to the Abiomed Impella ...
January 27, 2010 – A German study enrolled its first 20 patients to investigate the utility of muscular counter ...
January 26, 2010 – Surgical ventricular assist device (VAD) maker Thoratec Corp. acquired a catheter-based pump ...
January 21, 2010 – The FDA this week cleared the HeartMate II, a continuous-flow, left ventricular assist device ...
December 18, 2009 – Results from the Europella registry, evaluating the safety and feasibility of the Abiomed ...

Intra-aortic balloon pumps (IABPs), introduced in the 1970s, have been considered the standard of care for short ...
November 6, 2009 – Cardiac support device maker Abiomed Inc. yesterday announced second quarter fiscal 2010 ...
November 3, 2009 — CardiacAssist Inc. said yesterday the Cardiovascular Institute of Stanford University Hospital ...
October 22, 2009 – MAQUET Cardiovascular LLC said today it received European CE mark for the company's MEGA 8 Fr ...

 June 21, 2010
June 21, 2010