February 2, 2023 — Abbott today announced two approvals as part of its growing suite of electrophysiology products in the global market. The company’s TactiFlex Ablation Catheter, Sensor Enabled, the world’s only ablation catheter with a flexible tip and contact force sensing, received CE Mark1 for treating people with abnormal heart rhythms like atrial fibrillation (AFib). Abbott’s FlexAbility Ablation Catheter, Sensor Enabled also recently secured an expanded indication2 for treating patients with a complex heart condition by the U.S. Food and Drug Administration (FDA).
European Approval for New Catheter Opens New Doors for Afib Patients
Abbott received CE Mark of the TactiFlex Ablation Catheter, Sensor Enabled (SE), the world’s first ablation catheter designed with a unique flexible tip and contact force sensing, proven to reduce procedure times3 and patients’ exposure to radiation compared to standard power ablation4. When integrated with Abbott’s EnSite™ X EP System, which allows physicians to accurately identify areas in the heart that require an ablation treatment, the TactiFlex catheter can deliver high-power while more easily adapting to the heart tissue compared to conventional catheters5. The TactiFlex catheter can also result in reduced procedure times3 when compared to the company’s previous generation catheters4.
The European launch of TactiFlex is the latest within Abbott’s portfolio of electrophysiology solutions designed to better treat arrhythmias – especially around AFib, the most common arrhythmia that is a growing epidemic affecting 37 million people worldwide6. Initial cases leveraging TactiFlex occurred in the United Kingdom and Germany.
"When we treat complex ablation cases for people battling arrhythmias, we want to eliminate the arrhythmia and get our patients back to living their lives," said Isabel Deisenhofer, M.D., professor, head of the department of Electrophysiology at the German Heart Centre Munich in Germany. "The TactiFlex catheter’s data around using high-power during ablation will be game-changing for patients. When you combine these tools with Abbott’s EnSite X EP System, the innovation is truly opening new doors in patient care."
Millions of Europeans are affected by cardiac arrhythmias, caused by breakdowns in the electrical pathways of the heart that can lead to erratic heartbeats or cause the heart to beat too fast or too slow. AFib is a condition in which the heart's chambers are out of sync, causing them to beat in a rapid fashion. If left untreated, AFib may eventually lead to heart failure or stroke.
Physicians can perform an ablation to treat arrhythmias, in which long flexible tools—called catheters—are inserted into the heart to study and treat the arrhythmia. The catheters deliver radiofrequency (RF) energy to disrupt the tissue in the heart responsible for creating the abnormal heart rhythm.
Abbott’s TactiFlex catheter uses a tip design with a laser-cut pattern that flexes when in contact with the heart wall to direct irrigation flow to the treated tissue7 and to increase catheter stability by up to two-times for consistent therapy delivery5.
The catheter generated strong clinical outcomes in the TactiFlex AF IDE study for its treatment using high-power ablation (between 40 and 50 Watts)8. The study showed the catheter created fast, safe lesions to treat the patient’s arrhythmia the first time with over 99% acute procedural success3.
Abbott’s TactiFlex catheter is now available in Europe, Africa, Japan and Australia. It is currently undergoing FDA review for pre-market approval.
New U.S. Expanded Indication for Catheter Designed to Treat Complex Heart Condition
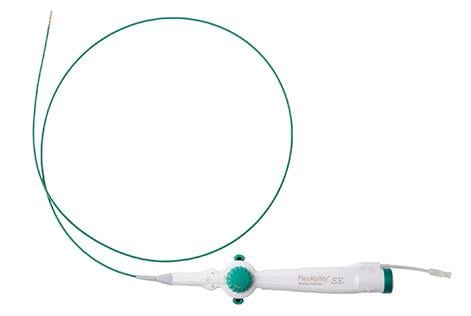
The company also received FDA approval for an expanded indication of its FlexAbility Ablation Catheter, Sensor Enabled (SE), a flexible tip catheter that helps physicians identify abnormal signals and apply therapy to treat a complex heart condition known as ventricular tachycardia (VT) in patients with non-ischemic cardiomyopathy (NICM). NICM is a type of heart muscle disease that prevents the heart from pumping blood effectively. This is associated with VT, a fast heart rhythm that can lead to cardiac arrest if untreated. Procedures to treat these patients are considered complex due to the nature of the disease itself and the need to treat both inside and outside surfaces of the heart.
Abbott’s LESS-VT study was the first FDA-approved pre-market trial to study the safety and effectiveness of ablation for the treatment of VT with NICM origin. Once treated with the FlexAbility Ablation Catheter, SE, 80% of study patients were free from VT for at least six months post-procedure9. The data also showed statistically significant improvements in patients’ mental and physical quality-of-life measures9.
For more information: www.abbott.com
References:
1 EU Indication for TactiFlex: The TactiFlex Ablation Catheter, Sensor Enabled is indicated for use in creating focal lesions during cardiac ablation procedures (mapping, stimulation, and ablation) for the treatment of arrhythmias. Epicardial ablation should be limited to appropriately selected patients with ventricular tachycardia.
2 The FlexAbility Ablation Catheter, Sensor Enabled, when used in conjunction with a compatible irrigation pump and compatible RF generator, is indicated for: Endocardial mapping, stimulation, and ablation for the treatment of typical atrial flutter. Endocardial or epicardial mapping, stimulation and ablation for the treatment of recurrent, drug-refractory, sustained monomorphic ventricular tachycardia in patients with non-ischemic structural heart disease, when used in conjunction with a compatible cardiac mapping system.
3 CL1017540 TactiFlex PAF IDE PMA Report
4 Lo MY, Sanders P, Sommer P, Kalman JM, Siddiqui UR, Sundaram S, Piorkowski C, Olson N, Madej SM, Gibson DN. Safety and effectiveness of a next-generation contact force catheter: results of the TactiSense trial. Clinical Electrophysiology. 2021 Aug 1;7(8):1013-21.
5 Compared to conventional 56-hole catheters. Ambrosius Nick, Fish Jeffrey, & Tranter John. Flexible, Kerfed Ablation Catheter Tip Provides Superior Stability in a Bench Model APHRS 2018: Abstract Book; 2018, October 17-18; Taipei, Taiwan. Abstract nr 1170.
6 Lippi G, Sanchis-Gomar F, Cervellin G. Global epidemiology of atrial fibrillation: An increasing epidemic and public health challenge. Int J Stroke. 2021 Feb;16(2):217-221. doi: 10.1177/1747493019897870. Epub 2020 Jan 19. Erratum in: Int J Stroke. 2020 Jan 28;:1747493020905964. PMID: 31955707.
7 Abbott data on file; 90906650
8 CL1019990 TactiFlex PAF IDE As Treated Repeat Procedure Details
9 CL1018185 LESS-VT NICM PMA Report


 October 28, 2025
October 28, 2025 









