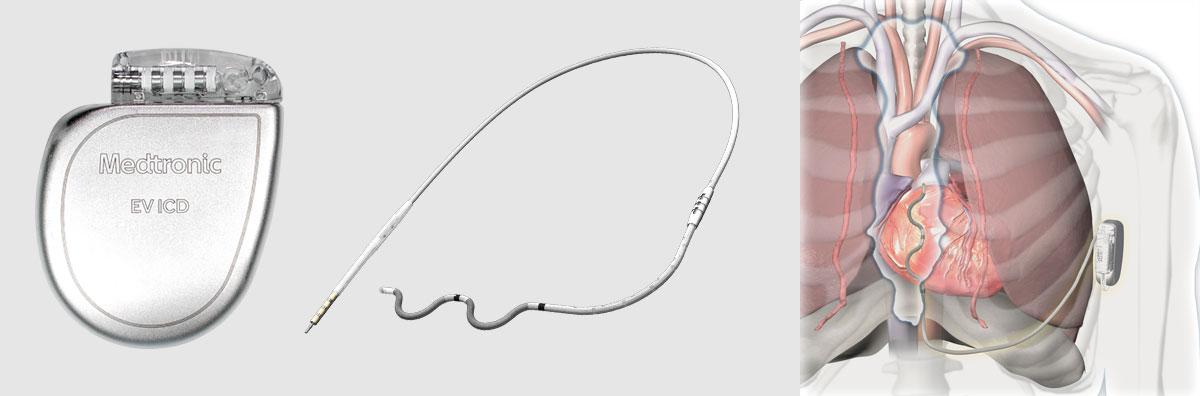
August 29, 2022 — Medtronic plc announced that its investigational EV ICD System – a first-of-its-kind defibrillator with the lead placed under the breastbone, outside of the heart and veins – achieved a defibrillation success rate of 98.7% and met its safety endpoints in a global clinical trial. Findings from the Extravascular Implantable Cardioverter Defibrillator (EV ICD) Pivotal Study were presented as late-breaking science today at the European Society of Cardiology (ESC) Congress 2022 in Barcelona and simultaneously published in The New England Journal of Medicine. Worldwide, the EV ICD system is investigational and not yet approved for sale or distribution.
The Medtronic EV ICD system is designed to treat dangerously fast heart rhythms that can lead to sudden cardiac arrest (SCA), while avoiding certain risks of traditional, transvenous ICDs because its lead (thin wire) is placed outside the heart and veins, under the breastbone (sternum) using a minimally invasive approach. Placing the lead in this location is designed to help avoid long-term complications that may be associated with leads in the heart and veins, such as vessel occlusion (narrowing, blockage or compression of a vein) and risks for blood infections.
The lead is connected to a device that is implanted below the left armpit (in the left mid-axillary region). Clinical trial participants received the same therapies provided by traditional ICDs, including defibrillation, anti-tachycardia pacing (ATP), and back-up pacing therapies with this single implanted device that is similar in size, shape, and longevity to traditional ICDs.
“We are very encouraged by the high defibrillation effectiveness and strong safety profile of the EV ICD system seen in the study, as we look to deliver less-invasive treatment options for patients at risk of sudden cardiac arrest,” said Ian Crozier, MB, CHB, M.D., Christchurch Hospital, Christchurch, New Zealand, who presented the results at ESC Congress 2022. “These results demonstrate the potential for this novel technology to be used as a safe, successful approach for patients with life-threatening arrhythmias.”
Study Results: Effectiveness
In the study, the device’s effectiveness in delivering defibrillation therapy at implant was 98.7% (298 of 302 patients), surpassing the prespecified performance goal of 88%. These results reflect a greater defibrillation efficacy for the EV ICD than historical transvenous ICD studies;1-4 comparable efficacy to the subcutaneous ICD despite EV ICD’s smaller device size;5 and a predicted increase in longevity compared to the subcutaneous ICD. Additionally, all discrete spontaneous arrhythmias were successfully treated (18 of 18, 100%).
Further, the efficacy of ATP – which paces the heart to interrupt and terminate a dangerous rhythm, potentially avoiding a defibrillation shock – in the EV ICD study was comparable to ATP efficacy in transvenous defibrillators.6,7 In total, 33 shocks were avoided by having ATP programmed “on.”
Study Results: Safety
The study also exceeded its safety endpoint: at six months, 92.6% of patients (Kaplan-Meier estimate) were free from major system and/or procedure-related major complications such as hospitalization, system revision, or death (compared to the performance goal of 79%; p<0.001). There were no major intraprocedural complications, nor any unique complications observed related to the EV ICD procedure or system (compared to transvenous and subcutaneous ICDs).
At six months, 25 major complications were observed in 23 of 316 patients who underwent an implant attempt (7.3%). Twenty-nine patients experienced inappropriate shocks (9.7%, average 10.6 months follow up), most commonly due to P-wave oversensing, which was more frequent in patients implanted early in the study and less frequent among patients implanted later in the study.
“These pivotal data mark the start of a new era in ICD therapy for patients who are at significant risk of dangerously fast heart rhythms,” said
, M.D., chief medical officer of the Cardiac Rhythm Management business, which is part of the Cardiovascular Portfolio at Medtronic. “Today’s findings are an important clinical milestone toward our goal of delivering a one-system, one-procedure extravascular ICD solution that prevents sudden cardiac arrest while improving the patient experience with a smaller device and moving the lead out of the veins and placing it under the breastbone. The EV ICD system retains the benefits of a completely extravascular system while providing ATP, pause prevention pacing and low defibrillation energy.”
The EV ICD Pivotal study is a prospective, multicenter, single-arm, non-randomized, pre-market clinical study that assessed the safety and effectiveness of the Medtronic EV ICD system for patients at risk of sudden cardiac death. The EV ICD Pivotal study enrolled 356 patients at 46 sites in 17 countries in North America, Europe, the Middle East, Asia, Australia and New Zealand. Medtronic has received FDA approval for a Continued Access Study while the agency reviews the company’s EV ICD pre-market application.
For more information: www.medtronic.com
More news from the European Society of Cardiology (ESC) 2022
Related content:
Medtronic Initiates Worldwide Pivotal Study of Extravascular Implantable Cardioverter Defibrillator
The Future of Cardiac Rhythm Management Device Technology
New Technology and Market Challenges Facing Implantable Cardioverter Defibrillators
Wearable External Defibrillator Offers a Bridge Therapy
Advances in Implantable Cardioverter Defibrillator (ICD) Technology
What is New in Electrophysiology Technologies
VIDEO: Overview of Subcutaneous ICD Technology — Interview with Lucas Boersma, M.D.
VIDEO: Advances in Electrophysiology Technology — Interview with Michael Gold, M.D.


 August 29, 2025
August 29, 2025